Bone, Live and Dynamic
Bone Tissue
Living Bone
Human bones in the laboratory are only a shadow of themselves as living bone tissue. This makes remembering bone names, without knowing the reason for such names, a challenge for most students new to the anatomy lab. The relevance of the subtle surface characteristics is seldom apparent. And imagining skeletal bones as living tissue with a network of nerves and blood vessels like other parts of the body requires creative thinking.
Live Bone Remodeling
Live bone remodeling is constant, even when dietary intake of calcium is optimal. Bone remodeling is a continuous process. The turnover rate for human bone segments is estimated to be 17 weeks. Approximately 10% of the human skeleton is replaced each year.
The purpose of bone remodeling is to replace damaged or fatigued bone with new mechanically sound bone. Remodeling also provides access to the skeleton’s reservoir of essential minerals to maintain mineral homeostasis. Under normal conditions two cell types, osteoblasts and osteoclasts, cooperate in the bone remodeling process. When there is a severe calcium deficiency a third cell type, osteocytes, also create a release calcium and phosphate from compact bone.
A large draw on plasma calcium like that during infant breast feeding activates osteocytes to reabsorb the bone of their lacunae. When calcium needs returns to normal, osteocytes restore the shape of their lacunae (shown in the image below).
Bone Microanatomy
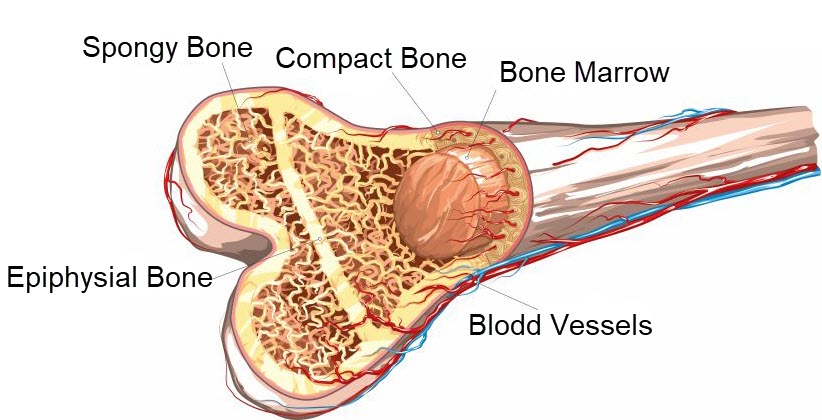
The microanatomy of compact bone differs from that of spongy bone as pictured below. Compact bone is built of cylindrical structures called osteons. Spongy bone assumes a spike like shape. In both types of bone the osteocytes live in lacunae, hollow spaces in the hard bone matrix. Photomicrographs from a light microscope show the difference in structure of the two types of bone.

Osteocytes in compact bone shown radially oriented around a central Haversian canal, Jose Luis Calvo, Shutterstock.com
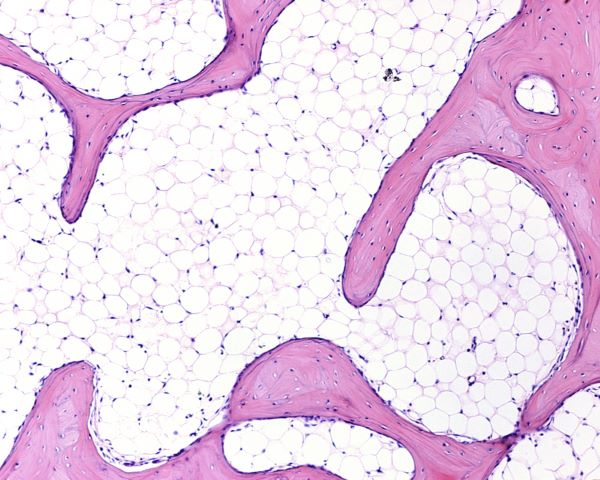
Light micrograph of network of spongy bone separated by spaces filled with adipose tissue, Jose Luis Calvo, Shutterstock.com
Bone Cell Types
Three major types of bone cells routinely maintain integrity of the skeleton: osteoblasts, osteocytes, and osteoclasts. Sometimes is hard to remember which bone cell does what job. It can help to focus on the more familiar meanings of the suffix terms after ‘osteo’. Osteo- always refers to bone. And ‘cyte’ refers to a cell –in this case a bone cell. The suffix ‘-blast’ comes from a Greek word that means to germinate, as in a seed germinating in spring. Germinating seed build a plant. The suffix ‘-clast’ comes from another Greek word meaning broken. Osteoclasts break down bone’s crystalline structure.
Osteoblasts
Osteoblasts are cells with a single nucleus that synthesize the calcium phosphate compound, hydroxyapatite to make bone hard. The matrix material in which they deposit hydroxyapatite is collagen, a much softer material which they also synthesize and secrete.
Osteoblasts operate in groups. Each group oversees building an osteon unit of bone. The osteoblast group synthesizes dense cross-linked collagen fibrils plus several other proteins needed in the bone matrix. They secret layers of collagen parallel to the long axis of the bone with alternating layers at right angles to the long axis. The cells in each osteoblast unit are linked to each other by tight junctions and gap junctions. Gap junctions are tiny pores that connect multiple cells such that they become one functional compartment.
Osteocytes
Osteocytes are former osteoblasts that become encased in the osteon that the osteoblast unit created. As osteoblasts become surrounded by bone, they begin to express different proteins and settle themselves into life as active bone regulatory cells.
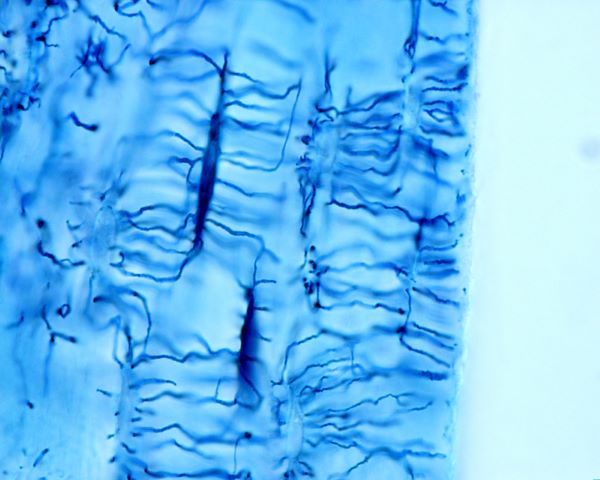
Osteocytes, dark blue, extending their plasma membrane though canals in hard bone matrix, Jose Luis Calvo, Shutterstock.com
Osteocytes are the most abundant cell type in bone, and they live as long as the section of bone they made. Even though they are totally encased in bone they are far from being isolated. The shape of these cells is dendritic, star shaped. They have long processes extending from a small round cell body. Osteocyte processes are extended, and they stretch through bony fluid-filled tunnels called canaliculi. The tips of the cell processes retain contact with the tips of processes of other osteocytes through gap junctions creating a network of bone-encased connected cells.
Extended osteocyte processes also contact the layers of cells lining bone surfaces, with osteoclasts, with blood vasculature, and with cells of the central bone marrow. The physiologic role of osteocytes was underestimated until very recently. Current research shows that osteocytes function as part of the regulatory network that controls the body’s calcium and phosphate homeostasis. Osteocytes affect bone remodeling by producing regulatory factors to influence the activity of osteoblasts and osteoclasts in response to endocrine signals including the blood level of vitamin D.
Osteoclasts
Osteoclasts are large, multi-nucleated cells found at the surface of both compact and trabecular bone. In histology sections under a microscope these cells are often located in surface pits that they created. Blood phagocytic monocytes, macrophages from the central bone marrow are the precursor of osteoclast cells.
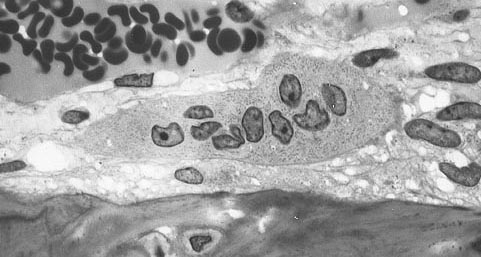
Multinucleated osteoclast Multinucleated Osteoclast near one (bone is at bottom of picture), Robert M Hunt released to public domain/Wikimedia Commons
In response to chemical signals from osteoblasts and osteocytes, osteoclasts develop from self-fusion of macrophages. Their phagocytic mechanisms are like to those of circulating macrophages. They secrete enzymes and acid that breaks apart the bone matrix. At a site of active bone resorption, osteoblasts can be identified by their ruffled cell membrane near the bone surface. Their cytoplasm is distinguished by its foamy appearance due to the many vesicles and vacuoles it contains.
Mechanical Stress on Bone
It is the osteocyte that senses mechanical stress on bone that is generated by strength and duration of muscle tension. Shear stress on bone transfers to the fluid surrounding osteocytes. Osteocytes have receptors that detect the fluid shear stress.
When osteocytes detect a change in mechanical strain on the skeleton, as during exercise, they secrete signaling molecules to osteoblasts and osteoclasts at the surface to stimulate osteoblast bone formation and to inhibit osteoclast bone resorption.
Adjusting to mechanical strain on the skeleton may widen the entire bone or change its axis by addition or removal of bone on appropriate surfaces. This process allows bone to dynamically maintain its most mechanically favorable architectural structure for strength, support, and protection of internal organs.
Bone Fracture Repair
Many cells participate in the bone fracture repair process. Cells from the periosteum, a fibrous layer of tissue on the external surface of bone, develop into osteoblasts. Other cells involved in the repair process come from small blood vessels, from the endosteum layer of cells covering the interior surface of bone, and from bone marrow when it is present.
Initial bleeding is slowed by blood vessel constriction. A blood clot forms and then some cells near the clot die freeing fibroblasts to replicate. The fibroblasts form a lose aggregate of cells and blood vessels called granulation tissue. Days after a fracture, cells of the periosteum replicate and become osteoblasts. Fibroblasts within the granulation tissue and some cells of the periosteum begin to secrete hyaline cartilage. Eventually the gap in the bone is bridged by hyaline cartilage and woven bone. At this stage some of the bone’s original strength is restored.
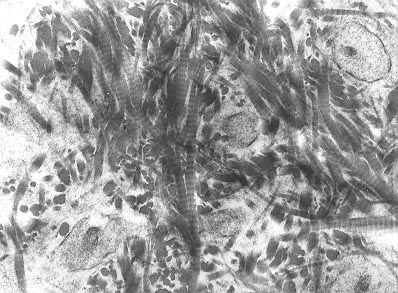
Early bone fracture repair tissue, woven bone matrix, Robert M Hunt released to public domain/Wikimedia Commons
Woven bone is replaced by trabecular bone. Later a remodeling process substitutes compact bone for the trabecular bone. The trabecular bone is reabsorbed by osteoclasts and the reabsorption pits are filled by osteoblasts. The complete remodeling process after a fracture takes 3 to 5 years depending upon a person’s age and general health. At the end of the process though, the bone closely duplicates its original shape and strength.
The next time you are in anatomy lab, look more closely at the human bone specimens. Notice the multitude of tiny holes in their surface that once served as channels for small blood vessels, nerves, and lymphatics. Inside those very small holes a vast colony of cells lived and connected with the rest of the soft tissue in the body through release of small regulatory molecules. Are there any signs that the bone had once been damaged and repaired?
Do you have questions?
Do you have questions about the physiology of living bone? Please put your questions in the comment box or send me an email at DrReece@MedicalScienceNavigator.com. I read and reply to all comments and email.
If you find this discussion of live bones helpful, share it with your fellow students or send it to your favorite social media by clicking one of the buttons below.
Further reading:
Hormone Regulation of Plasma Calcium: Human
Stem Cells: An Evolving Definition
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.



Pingback:Your Bones Are Alive! | Tammy Mundy, CMT, CZB
Pingback:Avoid Osteoporisis by Building Bone Strength and Flexibility - Zen and Vitality with Zoa