Physiology of the first trimester of human pregnancy
During the first trimester of human pregnancy, an ovarian follicle matures and ruptures, the haploid ovum is fertilized by sperm, a zygote forms, the zygote switches from meiotic to mitotic cell division, embryogenesis advances, and at week 11- gestation the embryo matures into a fetus.
To begin the story of the first trimester of human pregnancy, I will start where my previous article, ‘Spermatogenesis and Oogenesis’ ended. There an ovum/egg was waiting for fertilization and zygote formation. You can find that narrative by clicking here.
Human Zygote Formation
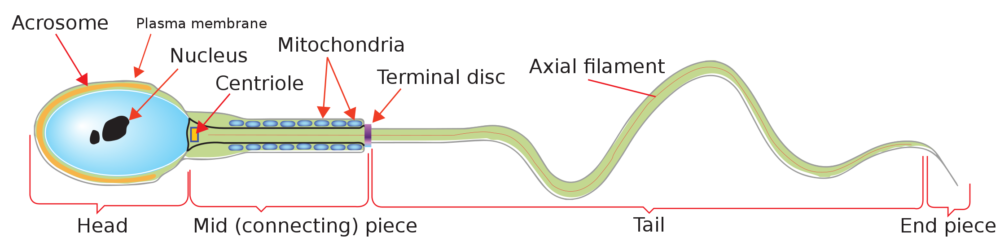
Human sperm anatomy
Human sperm anatomy is divided into functional sections that support successful fertilization. The rounded head of a human sperm cell is designed for penetration of the ovum. The central portion of the head piece contains cytoplasm and a haploid copy of the paternal chromosomes, tightly packed and sequestered in a nucleus. The acrosome, a vesicle surrounding the cytoplasm contains an array of enzymes needed to enter the ovum.
The middle piece of human sperm contains a centriole, mitochondria, and microtubules to assist in resumption of mitosis by the zygote. The tail and end piece form a flagellum, which propels the sperm through the female reproductive tract.
Female reproductive tract supports sperm viability
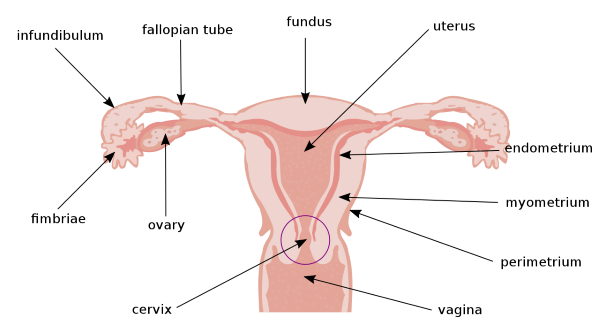
Parts of the female reproductive tract, Original: US Government Vector; Pixelsquid🎱, Public domain, via Wikimedia Commons
The female reproductive tract supports sperm viability, enhancing its ability to reach and fertilize the ovum. Menstrual cycle estrogen facilitates sperm interaction with the cells of the vagina, cervix, uterus, and fallopian tubes. Sperm can survive 3-5 days within the female reproductive tract.
When the dominant follicle ruptures, the ovum is collected by the fimbriae and deposited in the fallopian tube. During intercourse the maternal posterior pituitary releases the hormone oxytocin. Oxytocin causes contraction of the cervical, uterine, and fallopian tube muscles, propelling sperm toward the ovumfor fertilization
Sperm fertilization of the ovum
Sperm fertilization of the ovum occurs in the fallopian tube. The human ovum is a large cell, 20 um in diameter, arrested in metaphase of Meiosis II. It is surrounded by a gelatinous material called the zona pellucida, and it retains a layer of cumulus cells from the follicle. The cumulus cells help support fertilization.
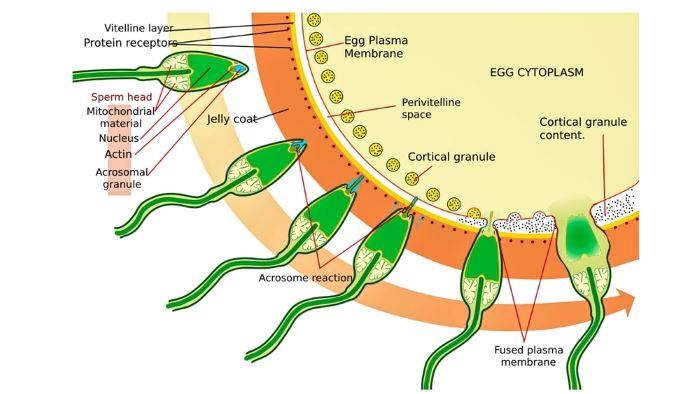
Stepwise entry of sperm into a human ovum, Original: US Government Vector: Pixelsquid🎱, Public domain, via Wikimedia Commons
This illustration shows how sperm (upper left) approaches an ovum’s zona pellucida (dark orange band). The following images of sperm show stepwise its penetration of the zona pellucida, an acrosome reaction, cortical granule release, and fusion of the sperm’s plasma membrane with that of the ovum.
The acrosome contains proteolytic enzymes that are released when the sperm contacts and binds to the zona pellucida. The enzymes facilitate penetration of the sperm head. When the sperm head reaches the ovum’s plasma membrane, it triggers ionic membrane currents that cause the cortical granules lining the plasma membrane to break open.
Opening of the cortical granules, ionic membrane currents, and fusion of the sperm head’s plasma membrane with the ovum’s plasma membrane all contribute to blocking entry of another sperm.
The sperm’s tail stops beating when the plasma membranes fuse. Ovum plasma membrane microvilli extend and draw the sperm into the ovum. The sperm nucleus and other organelles incorporate into the female cytoplasm. Cytoplasmic calcium ion (Ca++) oscillations initiate an ordered sequence of events that trigger completion of the ovum’s Meiosis II in what is now a zygote.
Ovarian meiotic method encourages genetic damage
The ovum accumulates genetic damage during meiosis. Meiosis, reduction of a diploid germ cell to a haploid cell, proceeds in the ovary with long pauses. The extended time course of ovarian meiosis affects the genetic quality of the zygote. The first stage of ovarian meiosis, Meiosis I, begins in the ovary’s germ cells during maternal fetal life.
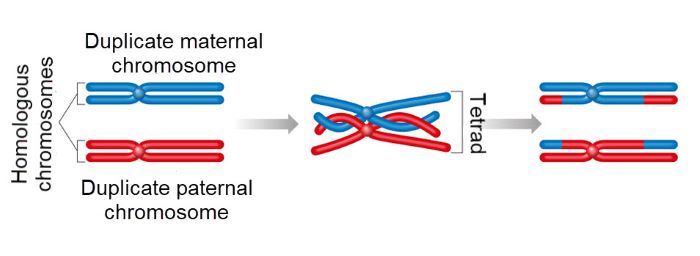
Recombination of parental chromosomes during prophase of meiotic cell division, Aldona Griskeviciene, Shutterstock
As oocytes mature, Meiosis I progresses through a prolonged prophase where chromosomes are doubled, and genetic material is exchanged between maternal and paternal homologous chromosomes. Then Meiosis I pauses for many years.
Resumption of Meiosis I occurs in the oocyte of the dominant follicle of each menstrual cycle at the time of the mid-cycle surge of luteinizing Hormone (LH). LH blocks inhibitory molecules within the follicular oocyte that stopped progress to Meiosis II. The surge of LH also causes rupture of the dominant follicle and release of the oocyte.
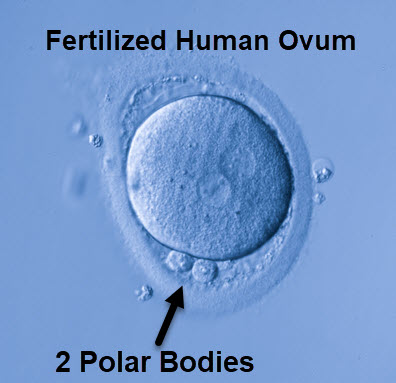
Following ovulation, the follicular oocyte becomes an ovum, or egg, and Meiosis I completes. Meiosis II begins and proceeds to the second metaphase, and the first polar body is released. The first polar body contains a small amount of cytoplasm and discarded chromosomes of Meiosis I.
Meiosis II remains at the second metaphase until a sperm penetrates the ovum. Following fertilization, Meiosis II resumes and a second polar body is released. The second polar body contains the 3 excess haploid female cells. The maternal and paternal pronuclei fuse.
The long pauses of female meiosis provide a period of years during which accidental genetic damage and aneuploidy of the ovum may occur. Aneuploidy is the presence of too many or too few chromosomes. In contrast, spermatogonia complete their meiosis within 3-4 weeks. Sperm are reported to be less aneuploid and to experience minimal random genetic damage.
Aneuploidy is present in 10-20% of eggs in women in their early 30’s, and in more than 50% of eggs in women over 40. Aneuploidy is a leading cause of miscarriages and infertility. Most aneuploid eggs cannot develop to term.
Aneuploidy is reported to rise to 50-70% during the mitotic cell divisions forming the human blastocyst, embryo, and fetus. Abnormal chromosomes impair the zygote’s switch from meiosis to mitosis for embryogenesis.
Zygote’s switch from meiosis to mitosis
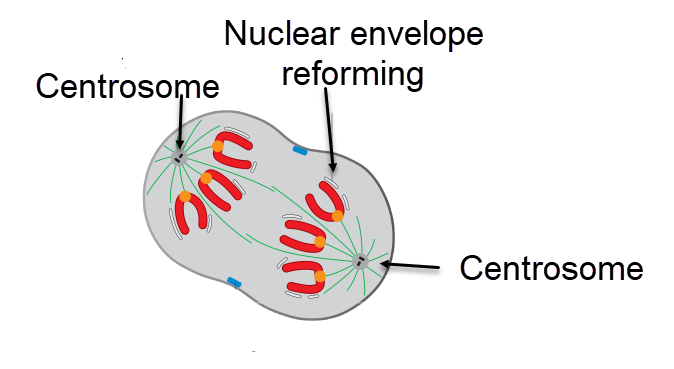
Telophase of mitotic cell divisions, equal daughter cells begin to form, David O Morgan, Attribution, via Wikimedia Commons
Specific events must occur for the zygote’s switch from meiosis to mitoses to advance. A mitotic spindle must be assembled. Meiosis in the egg uses a spindle apparatus that lacks centrosomes. For mitosis, centrosomes must be reintroduced, linkages between sister chromatids must be remodeled, and cell division must move toward the cell’s center for production of two equal daughter cells
Centrosomes are composed of two centrioles. Centrioles are a collection of tubules made with a protein named tubulin. The neck piece of human sperm (Refer to image above) contains one intact centriole, microtubules, and mitochondria to support the zygote’s construction of its first mitotic spindle.
Still, many more new proteins must be made to support mitotic cell division. Protein expression during meiosis is mainly by translation of mRNA stockpiled during the early oocyte’s growth phase. In the zygote, protein expression switches to transcription of nuclear DNA.
The first mitotic cell division of the zygote at 24-36 hours post fertilization begins embryogenesis, the transition from zygote to embryo.
Embryogenesis: Zygote Matures to an Embryo
Zygote to embryo, embryogenesis, happens during the three-week period from first cell division of the zygote to about day 23 post fertilization, which is the beginning of human gestation week 6. Because gestation of human pregnancy is timed from the first day of the last menstrual period, embryogenesis begins at the onset of gestation week 3.
Blastocyst formation
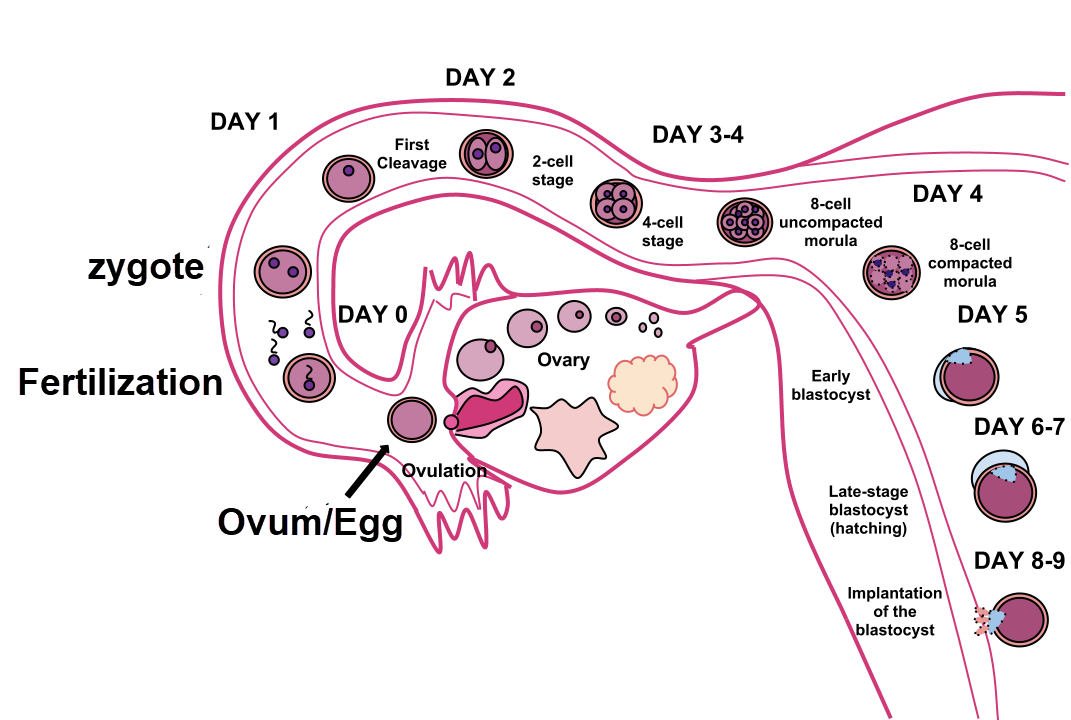
Dividing zygote travels through the fallopian tube to the uterus and implants, Ttrue12, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
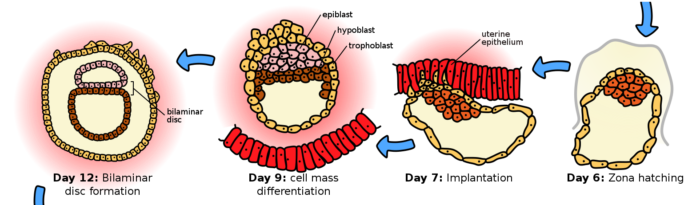
The zygote divides multiple times forming a blastocyst during gestation week 3. The dividing cell mass travels through the fallopian tube and into the uterus.
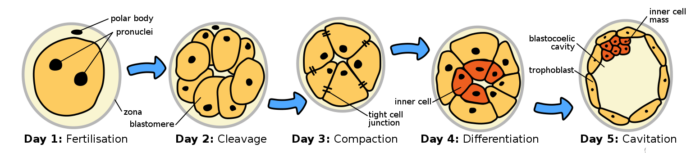
Day 1-5 post fertilization, gestation week 3 of human embryogenesis, Ttrue12, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
Blastocyst cells are much smaller than the fertilized ovum, and the early cell divisions are contained within the zona pellucida. By day 4 post fertilization, the first cell lineage decision is completed. The inner cell mass shown above in red later becomes the embryo proper. The outer cells in yellow, the trophoblast stem cells, become the placenta.
Blastocyte hatching and trophoblast formation, Ttrue12, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
At the end of the third week of gestation at about Day 6, the blastocyst hatches from the zona pellucida. The trophoblast cells are then able to infiltrate the uterine endometrium, implanting the nascent embryo.
About 30% of human blastocysts fail to implant or implant outside the uterus. Those that fail uterine implantation are thought to have an abnormal chromosomal karyotype that is sensed by the uterine endometrium. Losses of blastocysts at three weeks gestation are usually silent, because the cycle of menstrual periods is not noticeably disturbed.
Blastocysts that implant at sites outside the uterus, do so for a variety of reasons. Extrauterine blastocysts continue to grow but cannot mature into a viable fetus. Some misplaced blastocyst implantations occur in the fallopian tube. Untreated fallopian tube implantations are a leading cause of maternal death.
By day 12, during week 4 of gestation, two central rings form, one of trophoblast cells and one of cells of the inner mass. This is the first step in development of the germinal layers of the embryo and the accessory support structures needed for embryo maturation.
Embryo maturation
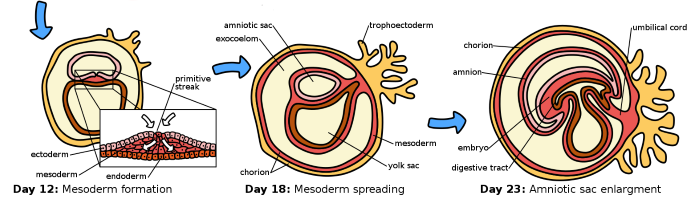
Gestation week 5 germinal layers of embryo form, ectoderm, mesoderm, and endoderm, Ttrue12, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
By the end of gestation week 4, Days 12-14 post fertilization, the inner cell mass divides into three sections which will give rise to the germinal layers of the embryo. During gestation week 5, the ectoderm, mesoderm, and endoderm layers are formed. Embryogenesis completes during gestation week 5, and its transition to a fetus begins with week 6.
The ectoderm produces neural tissues, spinal cord and nerve cells of the brain and periphery, and skin. The mesoderm develops into muscle, bones, ligaments, red blood cells, kidney tubules, and much of the reproductive system. The gastrointestinal tract, respiratory tract, endocrine organs, and the urinary system are all formed from embryonic endoderm. The developing placenta provides the embryo with a connection to the maternal circulatory system.
Developing placenta
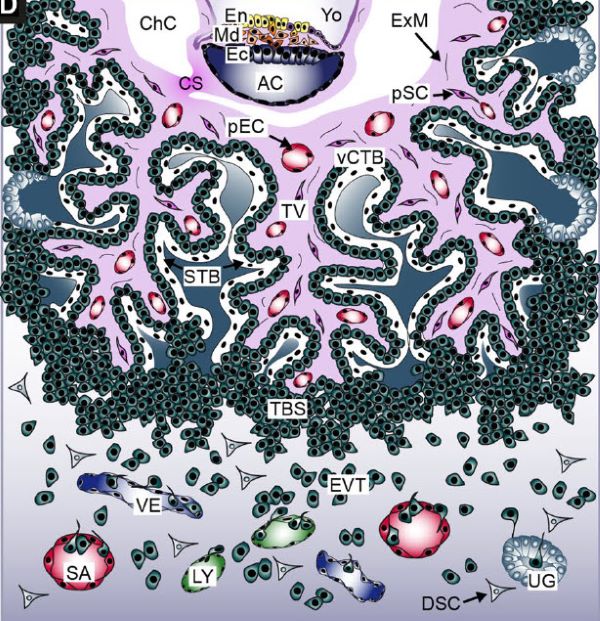
Extent of placenta infiltration of the uterus at gestational week 6, Martin Knöfler, et al. Cellular and Molecular Life Sciences (2019) 76:3479–3496, [Labels: En endoderm, Md mesoderm, and Ec ectoderm of the developing embryo, Yo yolk sac, ChC chorionic cavity] https://doi.org/10.1007/s00018-019-03104-6, Creative Commons — Attribution 4.0 International — CC BY 4.0
The placenta delivers nutrients, removes metabolic waste, and manages gas exchange for the embryo. The placenta also produces hormones and other factors that support fetal development. Failure of embryonic and/or placental development from gestation week 3 through gestation week 5 lead to an additional loss of about 30% of conceptions.
Human Embryo Transitions to a Fetus
The transition from embryo to fetus begins during gestation week 6, Days 21-28 post fertilization. Embryo to fetus transition continues from gestation week 6 to the beginning of gestation week 11. The transitioning embryo officially becomes described as a fetus at the beginning of gestation week 11. The first trimester of human pregnancy, the focus of this article, continues through gestation week 12.
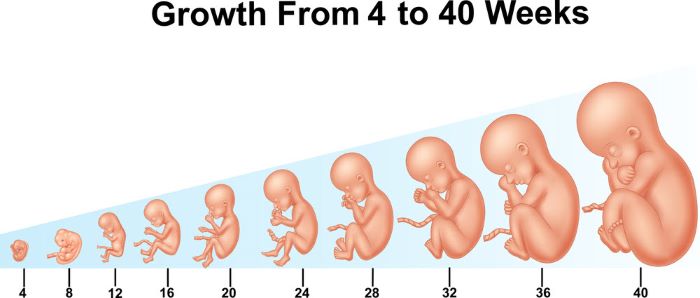
Relative size of the embryo and early fetus compared to that of a fetus at gestation week 40, https://www.shutterstock.com/g/Teguh+Mujiono, Shutterstock
Gestation week 6, neural tube closes
During gestation week 6, he neural tube along the back of the embryo closes. The neural tube eventually develops into the brain and spinal cord. Structures that will later become the heart and other organs are also just beginning to form during this week. Small surface buds appear that will become arms.
Gestation week 7, the head grows
During gestation week 7, the head begins to grow. A face emerges and the brain starts to develop. Facial depressions that later give rise to nostrils and retinas of the eyes appear. Lower limb buds emerge, and the arm buds of the previous week are shaped like paddles.
Gestation week 8, fingers begin to form
Eight weeks into pregnancy, 6 weeks past fertilization, the lower limbs continue to shape, and webbed fingers begin to form. Small swellings appear where ears will form. The eyes, upper lip, and nose form. The trunk and neck begin to straighten. By the end of gestation week 8, the transitioning embryo is about one-half inch long from crown to rump.
Gestation week 9, toes and eyelids visible
Toes and eyelids become visible during gestation week 9. The arms grow and elbows form. The head enlarges and the length of the transitioning embryo is about the same as the diameter of a US penny.
Gestation week 10, elbows can bend
During gestation week 10, the elbows can bend, and the fingers lose their webbing. The eyelids and external ears continue to develop. The umbilical cord connecting the placenta is now clearly visible.
Gestation week 11 gestation conceptus officially described as a fetus
At the beginning of week 11, week 9 after fertilization, the conceptus is officially described as a fetus. The head now makes up half of the fetal length. Buds for future teeth appear. Red blood cells begin to form in the fetal liver. By the end of the week, external genitalia will start developing.
Gestational week 12, fetal fingernails form
Gestation week 12completes the first trimester of human pregnancy. The face of the fetus displays a more developed appearance, and intestines have formed in the abdomen. The fetus grows to about 2 ½ inches and weighs about one-half ounce or 14 grams.
About 10% of fetuses that complete the first trimester of pregnancy fail to deliver an infant capable of survival. Some are lost to miscarriage, some are prematurely delivered, some die in the uterus, some are lost because the mother fails to thrive or survive.
Summary
The steps from fertilization of the human egg to delivery of an infant takes about 38 weeks. The first trimester of human pregnancy, zygote to embryo to fetus, is complicated by chromosomal aneuploidy, too many or too few chromosomes. The long pauses occurring during ovarian meiosis facilitates development of the egg’s aneuploidy. Failure of the switch from meiosis to mitosis by the zygote and poor placenta formation also contributes to the large loss of conceptus during the first trimester. Overall, the loss of conceptus by the end of gestation week 12 is about 60%. Fetal loss after the first trimester continues at a rate of about 10% for a variety of reasons.
Join my chat with Dr. Kevin Patton PhD, The A&P Professor, about the challenges of teaching reproductive anatomy and physiology by clicking here. You will learn why so little of what is in this post is found in your textbooks – yet.
Dr. Patton is the author of the textbook ‘Anatomy and Physiology of the Body’, now in its 16th edition. This is the 122nd episode of his podcast about teaching anatomy and physiology.
Please shared this with your social media following by clicking on one of icons on this page.
Prequel to this post:
Questions?
Send me an email with your questions at DrReece@MedicalScienceNavigator.com or put them in the comment box. I always read my mail and answer it.
 Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. For two decades she was a member of the science faculty in Obstetrics and Gynecology clinical departments. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by mail at DrReece@MedicalScienceNavigator.com.