Spermatogenesis and Oogenesis
Human hypothalamic – pituitary – gonadal axis after puberty
Puberty, spermatogenesis, and oogenesis is defined by the slow maturation of the components of the hypothalamic – pituitary – gonadal axis of hormone feedback loops. The story of reproductive endocrinology following puberty begins by describing the functional relationship of the hypothalamus and the anterior pituitary.
Hypothalamic Hormones
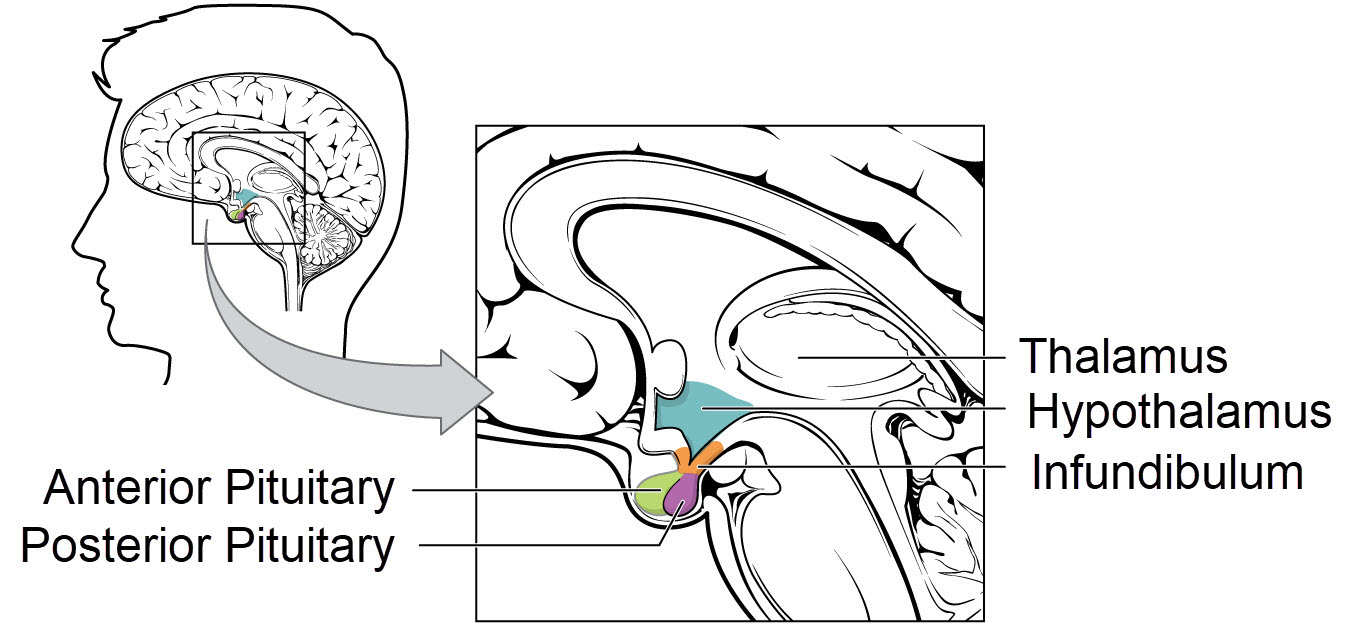
The hypothalamus sits at the base of the brain under the thalamus and near the brain stem. It is composed of a group of small brain nuclei. Remember brain nuclei are clusters of neuron cell bodies. Brain nuclei are the brain’s gray matter.
Some of the neurons of the hypothalamus are capable of releasing hormones into blood that directly affect distant target cells. That is, there are neuron secretions that fit the definition of a hormone.
One of the hypothalamic hormones is named gonadotropin releasing hormone, or GnRH. GnRH is picked up by the capillary bed of the infundibulum. The infundibulum is a thin stalk of tissue connecting the hypothalamus and the pituitary. Capillary GnRH is delivered to the cells of the anterior pituitary.
Because GnRH is a small molecule secreted into the capillary bed of a tiny bit of tissue, it was not isolated and characterized until 1971. It is a 10 amino acid peptide.
Human Anterior Pituitary
A human pituitary is divided into three parts, the pars distalis, the pars intermedia and the pars nervosa. The pars distalis is also commonly called the anterior pituitary. The pars nervosa is the posterior pituitary. Anterior and posterior pituitary are separated by the pars intermedia, which is normally inactive in adult humans.
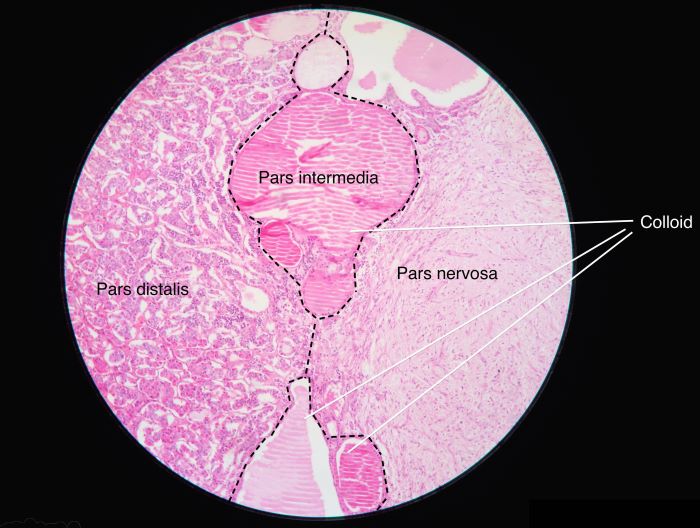
The pars distalis is also commonly called the anterior pituitary. The pars nervosa is the posterior pituitary, Athikhun.suw, Wikimedia Commons
Tropic Hormones
Tropic hormones that support ovarian and testicular function are synthesized in, and released from, the anterior pituitary.
Hormones secreted by the anterior pituitary are said to be ‘tropic’ because they are supportive of the function of other glands. The word tropic comes from a Greek root word that means nourishment or food.
Five types of tropic hormone-secreting cell populations are unevenly represented in the anterior pituitary. Gonadotropic hormones, those that support the ovary and testes, are produced by only 10% to 15% of the anterior pituitary’s cells.
Pituitary Gonadotrophs
The two gonadotropic hormones of the anterior pituitary are named luteinizing hormone, LH and follicle stimulating hormone, FSH. They were first described in females, but they are tropic to both the ovary and the testes.
The anterior pituitary releases LH and FSH simultaneously in response to hypothalamic gonadotropic releasing hormone GnRH. GnRH is released from hypothalamic neurons in a pulsate manner, making the secretion of LH and FSH also pulsate. The frequency of the GnRH pulses is regulated by hormonal feedback from the testes and ovaries.
Sperm Formation
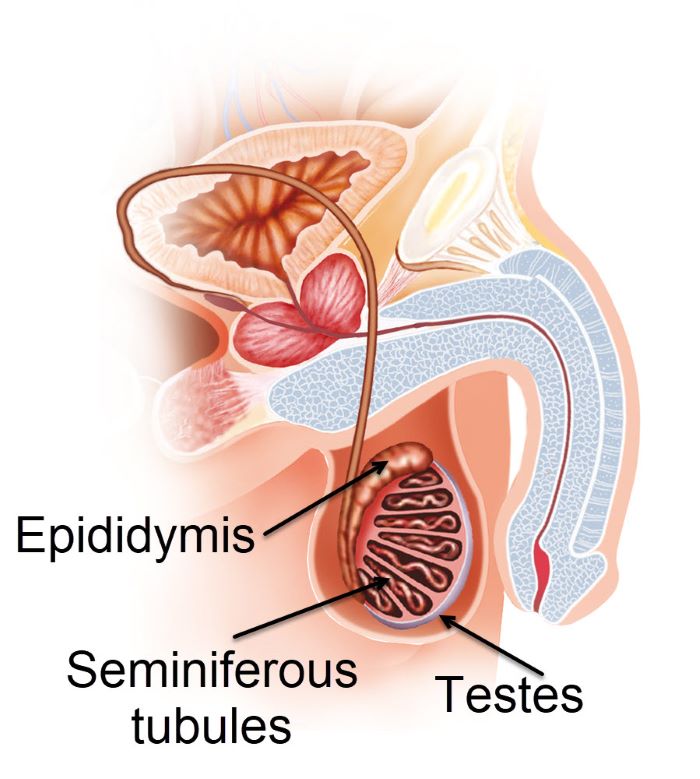
In the seminiferous tubules, under the influence of testosterone, spermatogonia (diploid male germ cells) undergo meiosis to reduce their chromosome complement to haploid. Haploid cells of the testes are called spermatozoa.
Spermatozoa move through the lumen of the seminiferous tubules into the epididymis where they mature into sperm and are stored until ejaculation.
Seminiferous Tubules
This photomicrograph shows the microscopic anatomy of the seminiferous tubules of a human testis.
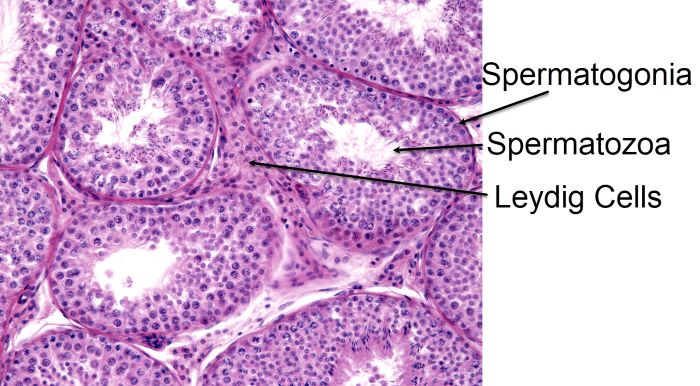
Spermatogonia, spermatocytes in meiosis, spermatids, and spermatozoa with their tails protruding into the lumen, Jose Luis Calvo/Shutterstock.com
At high power magnification, spermatogonia, spermatocytes in meiosis, spermatids, and spermatozoa can be distinguished. Tails of some spermatozoa extend into the lumen of the tubule.
The spermatogonia lie right under the outer cortex of the tubules. As the male germ cells undergo meiosis, they move toward the lumen of the tubule. Spermatozoa with tails are nearest the lumen. Spermatozoa swim into the lumen and travel through the tubule into the epididymis where they become mature sperm.
Leydig cells which secrete the male hormone testosterone are located between the seminiferous tubules. Testosterone is necessary for production of sperm, male secondary sex characteristics, and the masculine phenotype.
There is also another cell type within the seminiferous tubules that is difficult to distinguish in the image above. It is called a Sertoli cell. Sertoli cells function as nurse cells to the developing spermatogonia.
Sertoli cells
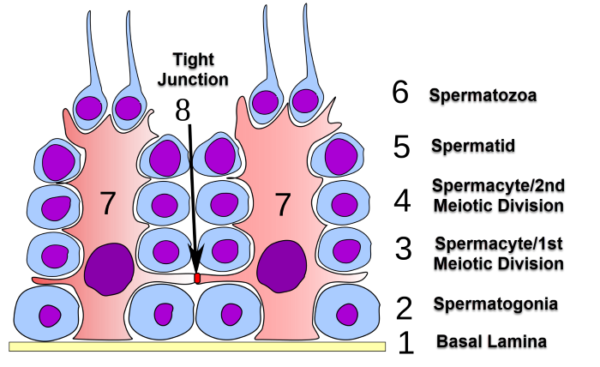
This illustration shows the anatomical relationship between Sertoli cells, labeled with the number 7, and the various stages of spermatogenesis within the seminiferous tubules.
Sertoli cells protect developing gametes from the host’s immune system with their tight junctions, tight association with the basal lamina, and the presence of little interstitial fluid.
The Sertoli cell barrier is sometimes called the blood-testes barrier. This barrier controls the entry of molecules from blood that may attack the male gametes as foreign entities.
It takes about 10 weeks for maturation of a spermatogonia to spermatozoa. Testosterone, secreted by the Leydig cells, activates Sertoli cell pathways supportive of the developing gametes.
Sertoli cells also secrete an androgen-binding protein into the seminiferous tubules to gather a high concentration testosterone. High testosterone in the tubules furthers development of spermatozoa into sperm in the epididymis.
Male hypothalamic – pituitary – gonadal – axis
Now that you know about the anatomic and functional relationship of the cells of the testes, it is time to talk about the hypothalamic – pituitary – gondal axis feedback loops. In males these feedback loops regulate testicular testosterone secretion.
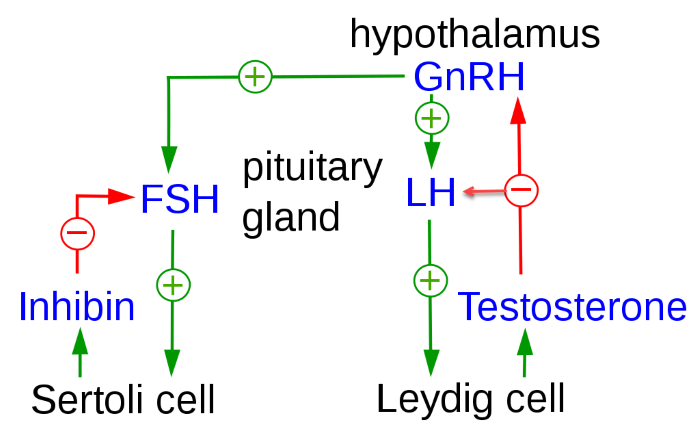
Hypothalamic – pituitary – gondal feedback loops that regulate testicular hormone secretion, Uwe Gilla/Wikimedia Commons
The frequency of GnRH pulses from the male hypothalamus is regulated by the level of testosterone in circulating blood. In the testes, LH and FSH are tropic to different cell populations, as shown in this illustration.
LH, luteinizing hormone is tropic to the Leydig cells and causes increased secretion of the male hormone testosterone. Ninety-five percent of circulating testosterone in males originates from the Leydig Cells.
Testosterone acts as a negative feedback molecule at both the anterior pituitary and the hypothalamus. When testosterone is sensed to be too high, it slows the pulsatile release of GnRH from the hypothalamus. The limited release of GnRH slows synthesis of LH by the pituitary.
In contrast to LH, follicle stimulating hormone FSH is tropic to the Sertoli cells. One effect of FSH at the Sertoli cells is its stimulation of the secretion of a hormone named inhibin. Inhibin is a negative regulator of the release of FSH from the anterior pituitary.
But inhibin is not a feedback regulator of hypothalamic GnRH release. Inhibin secretion by the Sertoli cells permits separate feedback adjustment of blood FSH and LH in males.
Ovum Formation – Human
Formation of a human ovum is more complex than production of a mature sperm. Ovaries of the female reproductive system periodically produce a single gamete that is ready for fertilization called an ovum. This contrasts with the male testes which continuously produces thousands of sperm.
Each menstrual cycle, one ovum is produced, and the uterus is primed for embryo implantation, Public Domain, US Centers for Disease Control/Wikimedia Commons
Females are only fertile during a short time window. The cycle producing an ovum lasts about 28 days. It is called the menstrual cycle. During each menstrual cycle, one ovum is produced, and the uterus is primed for implantation of an embryo.
If the ovum is not fertilized by sperm, an embryo does not form. Without an embryo, the prepared uterine lining degenerates, menses occurs, and a new cycle begins.
The Ovary
This photomicrograph displays the microscopic anatomy of a human ovary. The germinal epithelium lies just under the outer cortex.
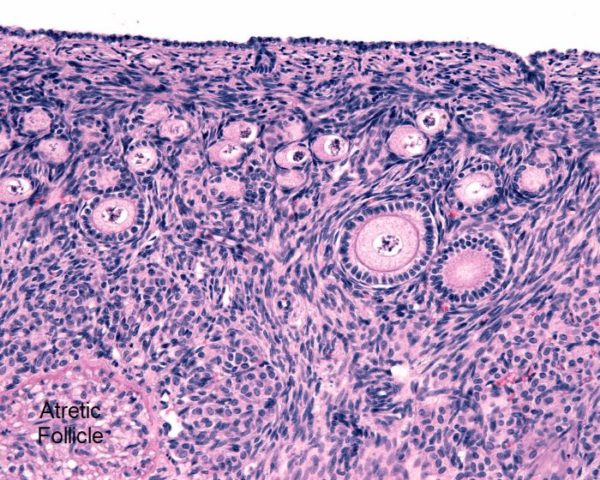
Below the germinal epithelium in this photomicrograph are several layers of primordial and primary follicles, Jose Luis Calvo/Shutterstock.com
During fetal development, the ovary gains a population of germ cells analogous to male spermatogonia. In the female they are named oogonia.
Below the germinal epithelium in this photomicrograph are several layers of primordial and primary follicles. Each contains a female germ cell at its center. At birth, human ovaries contain over 2 million primordial and primary follicles.
Primordial follicles are oogonia just beginning to acquire a coating of granulosa cells from the surround milieu. Primary follicles are surrounded by a layer of granulosa cells that feed the germ cell with macromolecules through gap junctions. The primary follicles have a central germ cell that has entered meiosis. Oogonia that have entered meiosis are called oocytes. More about oocyte meiosis is presented below.
By puberty, the number of primordial and primary follicles decreases to about 400,000. In the lower left corner of the image above, a dying, atretic follicle is labeled.
Ovarian Follicle Maturation
This illustration shows the stages of ovarian follicle maturation. The anatomic changes in the dominant ovarian follicle through a menstrual cycle begins with a primordial follicle.
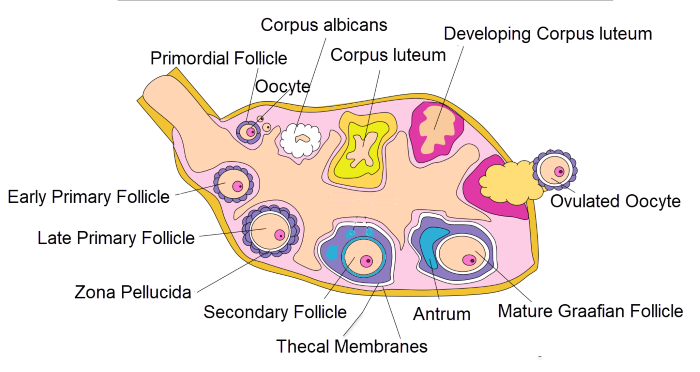
Anatomic changes in the dominant ovarian follicle through a menstrual cycle, Alexandra Garcia/Wikimedia Commons
A primordial follicle develops into a primary follicle as meiosis begins. The oocyte of the primary follicle enlarges, and it secretes a mucoid substance that separates it from the granulosa cells. This is represented by the clear area around the oocyte named the zona pellucida. The zona pellucida is labeled above in the late primary follicle.
During development of a primary follicle to a secondary follicle, the layers of granulosa cells multiply, and the oocyte enlarges to its mature volume. The granulosa cells of a secondary follicle recruit another outer layer of cells around the follicle to form the theca. A capillary network develops through the thecal cells. The granulosa cells and oocyte have no blood vessels.
As the ovarian follicle continues to grow into a mature Graafian follicle, the thecal cell layer expands, and a fluid filled cavity forms around the oocyte called an antrum. The liquid filling the enlarging antrum is blood plasma escaping the capillaries of the theca.
At the midpoint of the menstrual cycle, the Graafian follicle ruptures in response to a surge of pituitary luteinizing hormone, LH. The oocyte is expelled from the follicle, and the empty follicle transforms into a corpus luteum. The oocyte resumes and completes its Meiosis I, begins Meiosis II, and becomes an ovum.
If the ovum is not fertilized by sperm and a zygote (combination of ovum and sperm) formed, the cells of the corpus luteum die leaving a white scar in the ovary called a corpus albicans.
Oocyte meiosis does not complete until after the ovum is fertilized by sperm.
Oocyte Meiosis
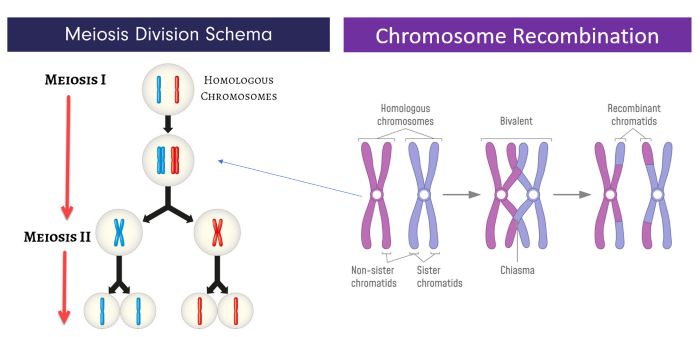
Meiosis I
Oocyte meiosis proceeds with long pauses. The first stage of meiosis, Meiosis I, begins during fetal life and arrests after doubling of the homologous parental chromosomes.
The above illustration on the left shows the general schema used in meiosis to reduce the diploid number of chromosomes to haploid (2N set to a 1N set). Human oogonia are diploid cells with 23 sets of homologous chromosomes. This illustration shows only one set of homologous chromosomes proceeding through meiosis for clarity.
Each of a set of homologous chromosomes originates from a parent, one from the mother and one from the father. During prophase of Meiosis I, the doubled homologous chromosomes, sister chromatids, remain attached. At this prolonged stage of Meiosis I, non-sister chromatids exchange genetic material as illustrated in the image above on the right. Chromosome recombination events ensures the haploid cells’ chromosomes will contain some genes from each parent.
After recombination events, Meiosis I in the oocyte pauses. The oocyte remains arrested at the diplotene stage of Meiosis I until a surge of LH from the anterior pituitary reaches the ovary at the midpoint of the menstrual cycle.
LH receptor activity at mid menstrual cycle causes rupture of the Graafian follicle and decreases the level of intracellular molecules that inhibited the next steps of meiosis in the follicle’s oocyte. After ovulation the oocyte becomes and ovum or egg.
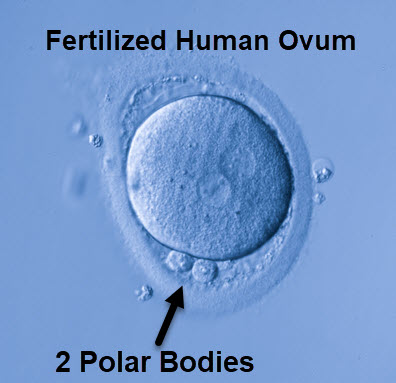
The ovulated ovum can then proceed through the first metaphase and separation of the homologous chromosomes but not of the sister chromatids. The set of chromosomes that does not go on to Meiosis II, are moved aside and extruded in a small structure with little cytoplasm named a polar body.
Meiosis II
Meiosis II begins in the ovum at the end of Meiosis I and continues to the second metaphase where it again pauses until fertilization by a sperm.
At fertilization, Meiosis II resumes separating the sister chromatids to produce 4 haploid cells. The last step in Meiosis II, dividing the genetic material into 4 haploid daughter cells occurs unevenly with one of the daughter cells retaining most of the cytoplasm.
The residual cytoplasm and genetic material of the 3 discarded daughter cells are expelled from the ovum and can be detected as the second microscopic polar body near the fertilized ovum. The female and male genetic material combine and cell division switches from meiosis to mitosis.
In a future article I will describe the first trimester of human pregnancy: Zygote to Embryo to Early Fetus. But now, I want to delve into the hormones that regulate female gametogenesis.
Hormones of Gametogenesis
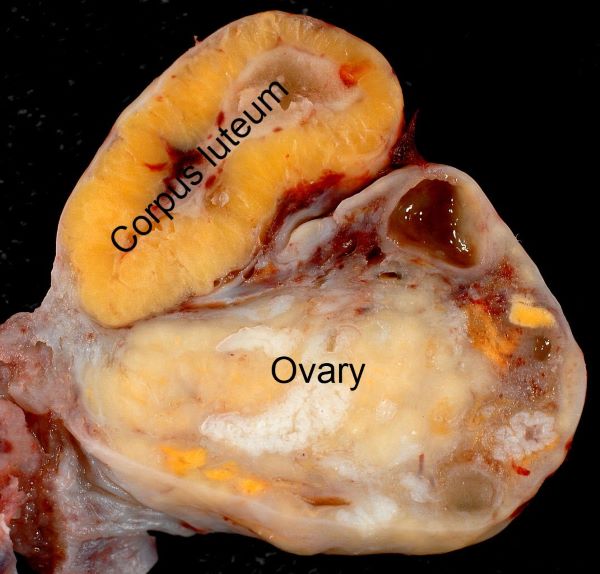
At ovulation, the remnant of the Graafian follicle becomes a corpus luteum. The microscopic anatomy of the mature Graafian follicle and corpus luteum are extremely different as you can see in these two photomicrographs.
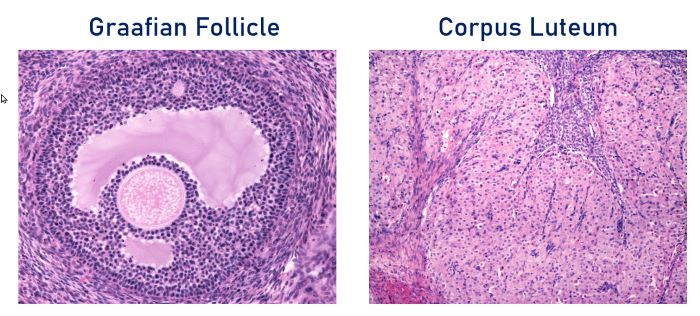
Corpus luteum develops from the thecal and granulosa cells of the Graafian follicle after ovulation, Jose Luis Calvo/Shutterstock.com
A Graafian follicle is highly organized, and the oocyte occupies a polar and off-center position. As the Graafian follicle enlarges, the oocyte retains a layer of granulosa cells surrounding it called the cumulus oophores.
Granulosa cells of the cumulus oophores remain attached to the oocyte at ovulation and provide substrates necessary for resumption of meiosis at fertilization. The cumulus oophores is also thought to aid in sperm penetration.
The corpus luteum develops from the thecal and granulosa cells of the follicle after ovulation. The corpus luteum viewed under a microscope is composed of a mixture of large and small cells. The follicular theca cells transform into small luteal cells and the granulosa cells transform into large luteal cells.
This is a picture of a corpus luteum on a human ovary about 10-12 days after ovulation.
While a mature Graafian follicle is large, a corpus luteum formed from it after ovulation dominates the ovary. A new corpus luteum is developed with each menstrual cycle, and it regularly measures between 2 and 5 centimeters. If a zygote implants in the uterus, the corpus luteum grows even larger, to form a corpus luteum of pregnancy.
Ovarian Hormone Secretion
Follicular Phase of the Menstrual Cycle
Now that you know the microscopic anatomy of the ovary, I can tell you about ovarian hormone secretion. I will start with estrogen production during the first half of the menstrual cycle.
The menstrual cycle is divided into two phases, the follicular phase before ovulation and the luteal phase after ovulation. During the follicular phase, a group of primary follicles develop into Graafian follicles. One of the Graafian follicles becomes the dominant follicle and proceeds to ovulation.
Pituitary FSH stimulates production of estrogen in the granulosa cells of the developing follicle by increasing their quantity of the enzyme aromatase. Aromatase is the enzyme that converts androgens to estrogens.
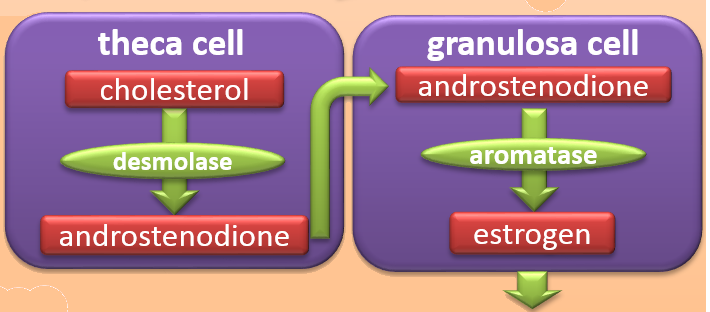
To make estrogen, the granulosa cells require help from the thecal cells, Isometrik/Wikimendia Commons
However, the granulosa cells do not have the other enzymes necessary to create androgen substrate molecules for the aromatase enzyme. To make estrogen, the granulosa cells require help from the thecal cells.
Thecal cells have LH receptors, and blood-borne pituitary LH during the follicular phase stimulates thecal production of androstenedione from blood cholesterol. The thecal cells deliver their androstenedione to the granulosa cells.
The granulosa cells convert the androstenedione into an estrogen named estradiol, and they secrete it into the general circulation. Estradiol stimulates the cells lining the uterus to proliferate and replace those lost during menstruation at the end of the previous cycle.
Luteal Phase of the Menstrual Cycle
During the second half of the cycle, the luteal phase, the ovary secretes a large quantity of progesterone and a small amount of estrogen.
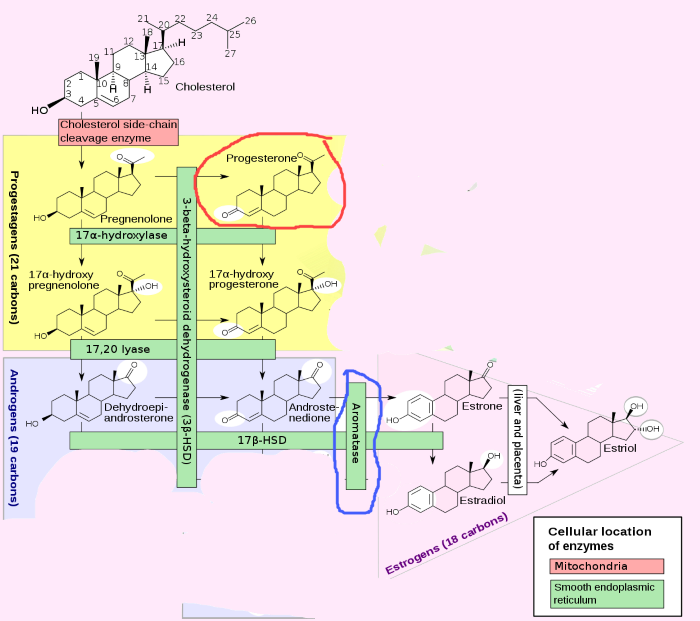
Let’s review how steroid hormones are made. This slide shows the enzymatic pathways needed to produce the hormones secreted by the ovary.
All steroid hormones are derivatives of cholesterol. The derivatives are made one step at a time. The aromatase enzyme, circled in blue, is necessary for production of estrogen.
And as you can see here, the precursor molecules for estrogen synthesis are androgen. Progesterone, circled in red, is made much earlier in the steroid synthesis pathway than androgen or estrogen.
The corpus luteum of the second half of the menstrual cycle is composed of modified thecal and granulosa cells. Luteal theca cells switch their primary hormone production from androstenedione to progesterone.
Luteal cells do, however, continue to produce a small amount of androstenedione. Luteal granulosa cells retain their aromatase activity and continue to convert the smaller amount of androgen to estrogen.
Ovarian Hormones and Uterine Endometrium
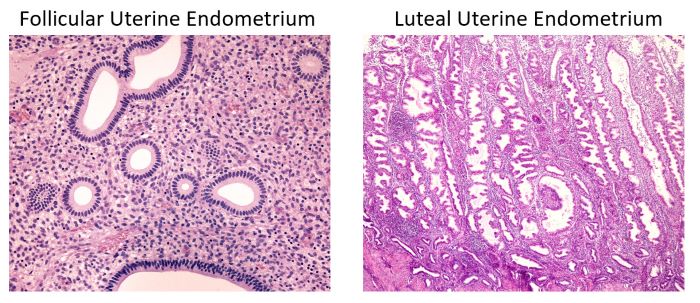
Estrogen stimulates follicular phase and progesterone luteal phase uterine endometrium, Jose Luis Calvo/Shutterstock.com
Ovarian hormones, estrogen and progesterone prepare the uterus for arrival of the zygote. When follicular estrogen rises in blood, the uterus develops a proliferative lining, an endometrium composed of simple tubular glands with one layer of columnar epithelium.
When luteal progesterone increases in blood, the endometrium expands forming large tortuous glands filled with secretory material.
If there is a zygote, the endometrial secretions of the luteal phase support the developing embryo and placenta formation. An embryo implanted in the uterine lining secretes its own hormone into blood called human chorionic gonadotropin.
Human chorionic gonadotropin signals the corpus luteum to continue its progesterone secretion. If chorionic gonadotropin does not appear in blood by 9-10 days after ovulation, the corpus luteum degenerates into a corpus albicans. The drop in luteal hormones causes the uterine endometrium to degenerate, and menses begins.
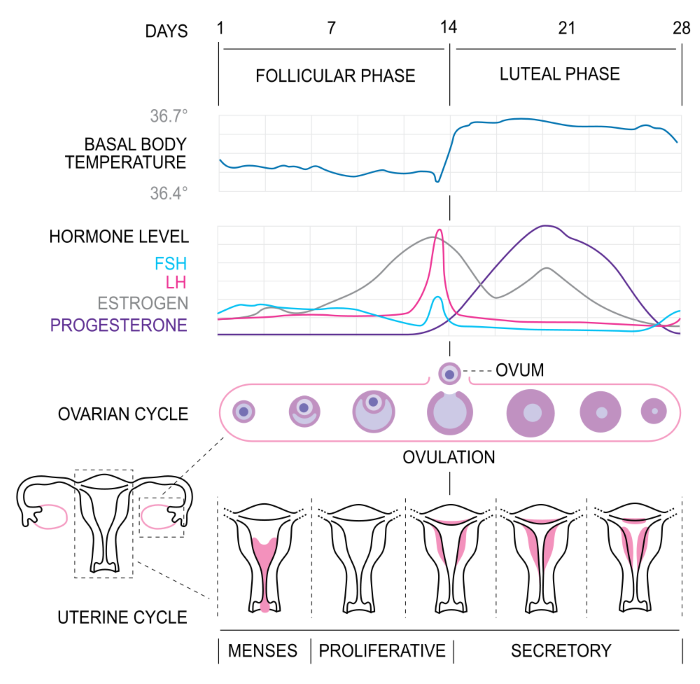
Summary: Menstrual Cycle Phases and Hormones
The top two sections of this illustration divide the days of the menstrual cycle into follicular phase and luteal phase and show a small, but predictable, shift in body temperature at midcycle.
The bottom two sections summarize events at the ovary and at the uterine lining. In the middle changes in blood hormones during the menstrual cycle are diagrammed. The hormone fluctuations shown are the result of both negative and positive hypothalamic – pituitary – gonadal feedback loops.
Follicular Phase Hormone Feedback Loops
Early Follicular Phase
Pituitary FSH and LH secretion is elevated in the early follicular phase because blood estrogen is exceptionally low at the end of the previous cycle. Early follicular phase FSH increases the amount of the enzyme aromatase in granulosa cells. In those follicles with a developed theca for production of androstenedione, the increase in aromatase activity boosts estrogen synthesis and secretion.
The rise in circulating estrogen, in turn, stimulates creation of hormone receptors for both FSH and LH in the plasma membrane of the granulosa cells, and receptors for LH on the thecal cells.
The continuous rise in follicular estrogen toward the end of the follicular phase has a negative feedback effect on FSH secretion at the anterior pituitary. But follicular estrogen also has another effect at the gonadotropic cells of the pituitary.
Late Follicular Phase
The long duration of high blood estrogen makes the FSH and LH secreting cells more sensitive to hypothalamic gonadotropin releasing hormone, GnRH. That is, estrogen increases the ability of the gonadotrophs’ receptors to respond to FSH and LH. This positive estrogen feedback effect causes a short, mid-cycle surge of LH and FSH in blood.
The positive feedback effect of estrogen at the gonadotrophs requires at least 2 days of high circulating estrogen. Such conditions only exist near the middle of the menstrual cycle.
The FSH surge is blunted by the high blood estrogen level at mid-cycle, but the LH surge is not. On this graph, the surge in blood LH is shown in magenta and the surge in FSH is shown in green.
The surge of blood LH is responsible for the subsequent release of the ovum from the dominant follicle and resumption of meiosis as discussed above.
Luteal Phase Feedback Loops
Ovulation marks the beginning of the luteal phase and corpus luteum development. The corpus luteum steroid synthetic pathways switch to high production of progesterone. Continuing low level estrogen secretion keeps LH and FSH levels minimal during the luteal phase.
If there is no fertilization of the ovum by sperm, the corpus luteum degenerates and progesterone and estrogen rapidly fall. Menstruation occurs with the drop in circulating progesterone to support the endometrial glands. With little circulating estrogen, the anterior pituitary is again able to secrete FSH and LH and begin the next menstrual cycle.
Fortunately, not all endocrine feedback loops are as complex as the human hypothalamic, pituitary, gonadal axis following puberty.
Further reading
Do you have questions?
Send me an email with your questions at DrReece@MedicalScienceNavigator.com or put them in the comment box. I always read my mail and answer it. Please share this article with your fellow students taking anatomy and physiology by clicking your favorite social media button.
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.


Comments
Spermatogenesis and Oogenesis — No Comments
HTML tags allowed in your comment: <a href="" title=""> <abbr title=""> <acronym title=""> <b> <blockquote cite=""> <cite> <code> <del datetime=""> <em> <i> <q cite=""> <s> <strike> <strong>