Blood pH: buffers, lungs, and kidneys
Acidosis vs. alkalosis
There is a constant production of acid in body tissues and fluids due to normal physiological processes such as breaking down of protein molecules and muscle energy use during contraction. Acidosis occurs in adults when pH of body tissues and of blood in the arteries falls below 7.35.

Muscle energy molecules used during running adds lactic acid to blood, Andrey Burmakin/Shutterstock.com
In contrast, alkalosis, an increased alkaline condition, is a circumstance where pH is elevated above 7.45 because the blood has lost too much hydrogen ion [H+]. One cause may be severe vomiting. Severe vomiting can result in loss of stomach acid [H+], potassium ion [K+], and sodium ion [Na+] along with the stomach contents. When this happens kidney processes compensate for the loss of K+ and Na+ at the expense of H+, leading to a condition called metabolic alkalosis.
In healthy people wide shifts in blood pH do not happen. Rapid response to both acidosis and alkalosis is managed by blood buffers and lung ventilation of CO2. Long term maintenance of neutral blood pH is accomplished by the kidney’s secretion of excess H+.
Blood buffers
Because ongoing cellular metabolic activity requires a steady local pH, the fast response to local changes in blood pH is managed by blood buffers. Buffers are a mixture of molecules that are part weak acid and part base.
An acid is any molecule soluble in water that breaks apart upon solution such that one fragment is an H+. The other fragment formed upon dissociation of an acid carries a negative charge similar to the OH– fragment of auto dissociated water. The negatively charged fragment of a dissociated acidic molecule is called a base.
There are many buffers used by the human body, but the most important one for maintaining neutral pH in blood is the carbonic acid – bicarbonate mixture.
Carbonic acid has the molecular formula H2CO3. Bicarbonate, a base, has the molecular formula HCO3–. A summary of reactions available to carbonic acid are shown by the reaction sequences below.
CO2 + H2O ↔ H2CO3 (carbonic acid)
H2CO3 ↔ HCO3– (bicarbonate) + H+ (hydrogen proton)
Arrows pointing forward and backward indicate that these reactions can proceed in either direction depending upon the availability of the components.
The hydrogen proton released by carbonic acid, one part of the buffering mixture, may combine with water to form hydronium ion [H3O+] making the solution more acidic. Or, it may attach itself to a basic molecule – either its own base fragment bicarbonate, or another basic molecule present in solution, or with OH–.
The second part of this buffering mixture, the base bicarbonate, is present in blood in greater quantity than carbonic acid. The amount of HCO3– in solution that is in excess to that formed by the dissociation of carbonic acid comes from other compounds such as sodium bicarbonate, NaHCO3. Sodium bicarbonate is a salt, and it is dissolved in water much like NaCl, table salt.
Lung ventilation
Lung ventilation affects the blood bicarbonate buffer. The left side of the chemical equation from above displays another valuable characteristic of the carbonic acid/bicarbonate buffering system. H2CO3 can be reversibly converted to CO2 and H2O.
CO2 + H2O ↔ H2CO3 (carbonic acid) ↔ HCO3– (bicarbonate) + H+ (hydrogen proton)
It is that part of the reaction sequence that makes this buffering system particularly flexible. Because all these reactions are reversible, removing CO2 from the left side of the equation – as when CO2 is ventilated from the lung – causes reformation of H2CO3 from HCO3– and H+ reducing the level of acid H+ in blood.
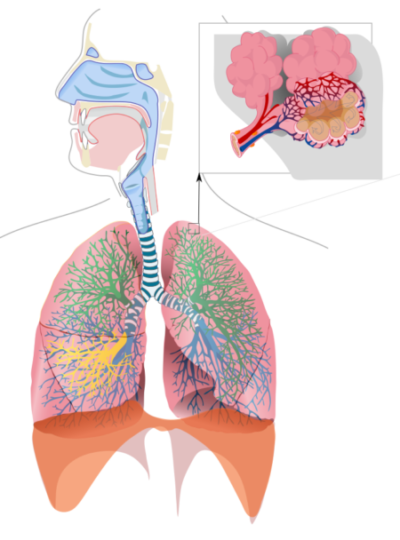
Respiratory system is an important regulator of blood pH, Released to Public Domain by LadyofHats/Wikimedia Commons
Increasing CO2 in the blood due to energy demands of tissue, as when muscle contracts, pushes the buffering reaction in the opposite direction. Carbon dioxide and water combine to form carbonic acid. Part of the carbonic acid dissociates into bicarbonate and H+.
This decreases blood pH and serves as a signal to central control centers to increase the rate of breathing. Increased breathing causes release of more carbon dioxide from the lung. As carbon dioxide is released into air, blood pH increases again toward neutral because the carbonic acid reactions proceed in the reverse order and breathing rate returns to baseline.
A more detailed explanation of blood buffers and how lung ventilation affects blood pH can be found in my book “Physiology: Custom-Designed Chemistry.”For a look inside click here.
Also, if you are interested in learning more about the physiological response to acidosis and alkalosis in a clinical setting check out Normal Acid-Base Regulation, a YouTube video created by Eric Strong MD, Stanford University.
Further reading
Physiology: Why Learn Its Chemistry Now?
Do you have questions?
Please put your questions in the comment box or send them to me by email at DrReece@MedicalScienceNavigator.com. I read and reply to all comments and email.
If you find this article useful to your understanding of medical physiology share it with your fellow students or send it to your favorite social media site by clicking on one of the buttons on this page.
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.