Stem-cell-like macrophages
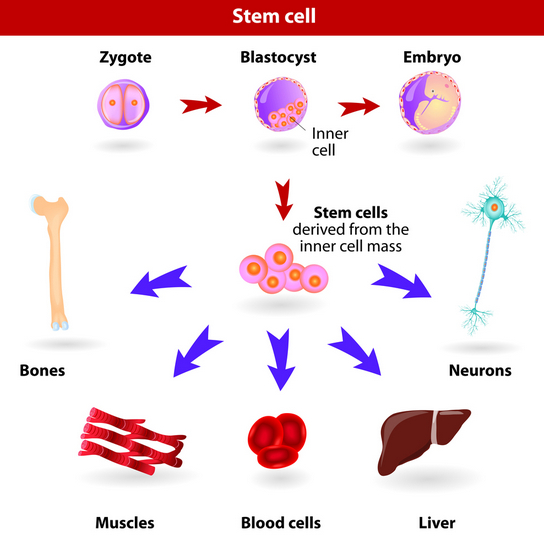
By definition, embryonic stem cells are undifferentiated cells that are capable of maturing into many different cell types depending upon their local environment. When stem cells divide, a portion of the new cells continue to mature into specialized cells. But, a portion of the new cells do not mature. They maintain their immature stem cell characteristics.
Until recently, scientific dogma was that completely mature, differentiated cells are unable to reproduce themselves to sustain their population. Specialized tissues were all thought to rely upon tissue-specific adult stem cells for their renewal and repair. Adult stem cells are partially matured stem cells committed to becoming specific tissues.
Stem cell definition
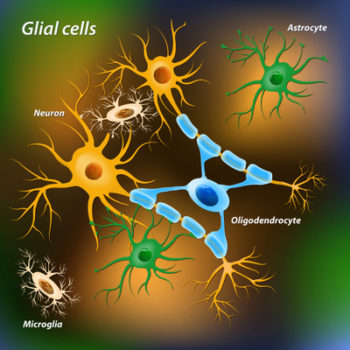
The long-accepted definition of stem cell is challenged by recent studies of tissue resident macrophages in most body tissues. One such population is brain specific macrophage-like cells named microglia.
Microglia is derived from embryonic progenitors in the yolk sac long before the appearance of the stem cells in bone marrow that replenish circulating white and red blood cells.
The brain possesses all the microglia it needs for a lifetime of defense at birth. Fully mature, differentiated microglia is able to massively expand it population under circumstances of stress and infection without the help of neural stem cells. The adult brain contains neural stem cells, but neural stem cells can only produce neurons, oligodendrocytes and astrocytes.
Immune system macrophages distributed in tissues throughout the rest of the body play a role in local metabolism and are essential to tissue repair, immunity against pathogens and cancer. They are phagocytic cells with high lysosome activity. They, like microglia of the brain, clean up debris as cells die.
Recent studies of a wide range of tissue macrophages from liver, skin, lung, spleen, adipose tissue, cardiac tissue, pleura and peritoneum demonstrate that the cell cycle withdrawal accompanying maturation of tissue macrophages can be reversed without them becoming tumorigenic.
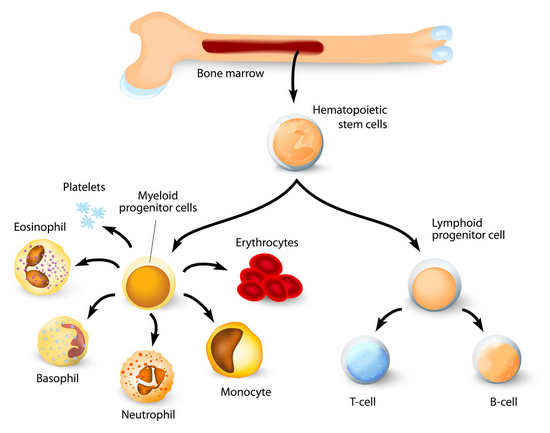
Older studies concluded all immune system macrophages developed from monocytes. Monocytes are partially mature bone marrow stem cells, adult stem cells by definition. Lineage tracing experiments now suggest many of adult macrophage populations are derived from embryonic progenitors rather than from bone marrow monocytes.
Embryonic stem cells
Body tissues are seeded during early embryonic development with fetal liver and yolk sac cells that develop into tissue-specific resident macrophages. These cells occupy the tissue before bone marrow blood formation begins. Unlike blood monocytes that die in a few days embryonic tissue macrophages can persist throughout a lifetime.
Each tissue induces special properties in its macrophage population. Like other stem cells, tissue macrophages respond to their local environment. Also like other stem cells, tissue macrophages establish and maintain their population size by reentering the cell cycle to reproduce themselves. Proliferation sustains the population under homeostatic conditions and replenishes it after severe depletion by stressful conditions and infection.
Embryonic macrophage stem cells mature into macrophages under stress. They are the first respondents to infection and are the tissue’s first line of defense. They react by massively proliferating, maturing and attacking pathogens.
Monocytes also defend against a broad range of pathogens, but they enter tissues during infection from the blood stream. Once in the tissue they transform into macrophages to assist the tissue macrophages destroy the intruders. Monocyte derived macrophages are important to finishing the kill and repairing the damage.
With severe infection there is a great loss of the tissue’s embryonic macrophage stem cells. Recovery of the embryonic stem cell population requires expansion of the remaining mature macrophages of embryonic origin. There is little to no permanent rebuilding of the population by macrophages of monocyte descent.
Transcription factor programs for reading out DNA of the two types of macrophages, embryonic and monocyte derived, are under active investigation. Early results indicate that regulation of molecular pathways is different in these two types of macrophages.
It is of therapeutic importance to determine whether regulation of embryonic tissue macrophages, an accessible population, is like regulation of brain microglia. Brain microglia experiments are hampered by the blood brain barrier. Yet, many degenerative conditions of the brain, including Alzheimer’s disease, have a strong, unfavorable microglial inflammatory component.
Further reading:
Microglia Maintenance of Neuron Synapses
Do you have questions?
Do you want to know more about the definition of stem cells? Please put your questions in the comment box or send me an email at DrReece@MedicalScienceNavigator.com. I read and reply to all comments and email.
If you think this description of stem cells is helpful, share it with your fellow students or send it to your favorite social media by clicking one of the buttons below.
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.