3 Ways ependymal cells nurture the brain
1. Cerebrospinal Fluid
Cerebrospinal fluid produced by ependymal cells provides both mechanical protection – padding – and a means to remove metabolic waste products – garbage disposal. The brain and spinal column are composed of very soft tissue and need to exist in a wet environment that cushions them within the protective bone of the skull. And with the blood brain barrier in place, it provides an alternate path for waste disposal.
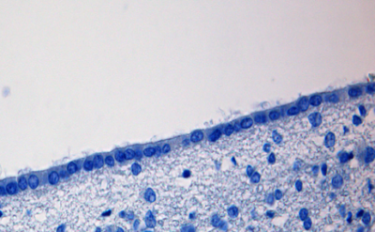
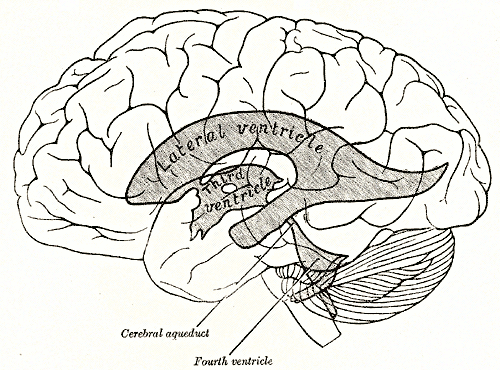
Ependymal cells are cube-shaped epithelial cells that line the surface of the brain’s four interior chambers, the ventricles, and the central canal of the spinal cord. Ependymal cells secrete cerebrospinal fluid and absorb it. The surface of the ependymal cell layer that faces the ventricles is covered by cilia and microvilli. Whip like motion of the cilia stir and move the cerebrospinal fluid in the ventricles. Uptake of cerebrospinal fluid by microvilli permits ependymal cells to monitor the quality of the cerebrospinal fluid and provide underlying brain tissue with widespread access to cerebrospinal fluid proteins.
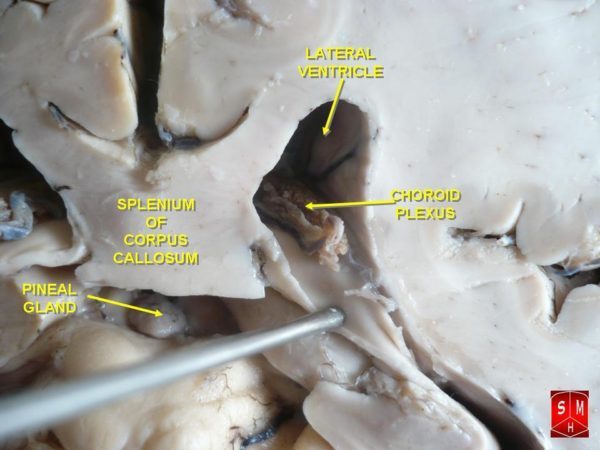
Modified ependymal cells that are continuous with the ventricular ependymal cell layer form the outer covering of a ventricle-wall structure called the choroid plexus. The choroid plexus consists of many capillaries separated from the cavity of the ventricle by the modified ependymal cell layer. It is the choroid plexus that produces most of the cerebrospinal fluid.
Fluid filters from the choroid plexus capillaries through the ependymal cell layer to become cerebrospinal fluid. To increase the efficiency of this process, choroid plexus ependymal cells actively transport sodium, chloride, and bicarbonate ions into the ventricles to create an osmotic gradient that continuously draws capillary water across the cells and into the cerebrospinal fluid.
There are four choroid plexuses in the human brain – one in each ventricle. The entire volume of cerebrospinal fluid is recycled back into the blood about 4 times per day to remove metabolic waste products and excess neurotransmitters that find their way into the fluid. In humans, about 500 milliliters of cerebrospinal fluid is produced by the choroid plexuses each day.
2. Neural Stem Cells
The presence in the adult brain of neural stem cells is an exciting area of current research. For a long time it was thought that nervous system plasticity – the reciprocal interaction between brain structure and brain function – only involved rearrangement of the contacts between pre-existing ‘old’ neurons. That view is changing to include neurogenesis, the birth of new neurons, in an ongoing process of adult brain plasticity, even in the human brain.
In adult rodents and in non-human primates there is a zone of cells below the ependymal epithelial cell layer in the lateral ventricles of adult animals that serves as a reservoir of neural stem cells. Neural stem cells are a potential source of new neurons for repair of brain injuries. In humans the existence of this cell layer is of great interest but still controversial. This is because confirmatory studies of a similar reservoir in humans using autopsy tissue have produced mixed results.
Studies using adult mice estimate that about 10,000 new neurons are generated daily from sub-ependymal layer neural stem cells. Most of these progenitor cells in mice migrate and differentiate into various types of interneurons in the olfactory bulb.
Ependymal cells lining the ventricles maintain the structural integrity of sub-ependymal layer reservoir and possibly provide metabolic support to developing stem cells. In mice the neural stem cells send a long membrane projection between ependymal cells to make direct contact with the ventricular fluid. Additionally, they send a second membrane projection to wrap around a capillary in the vicinity. Because of this anatomical arrangement, it is thought that activation of mouse neural stem cells depends upon a combination of signals from ependymal cells, the cerebrospinal fluid, and blood in the capillaries.
Using the mouse model, studies focused upon the origin of adult neural stem cells of the sub-ependymal layer have also produced a new understanding of the common origin of macroglia and neurons. Macroglia, astrocytes and oligodendrocytes, were long considered specialized supportive cells with an embryonic origin very different from that of neurons.
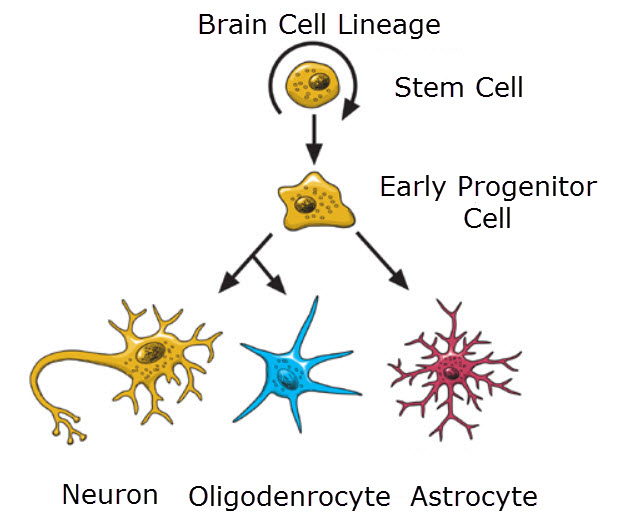
Neurons, oligodendrocytes and astrocyes all develop from the same neural stem cell, National Institutes of Health United States/Wikimedia Commons
However, new studies demonstrate that astrocytes, oligodendrocytes and neurons arise from a common stem cell during embryogenesis. Furthermore, one type of adult astrocyte, the radial astrocyte, is the adult neural stem cell. Macroglia and neurons only represent different stages in development of a single embryonic cell type.
The physiology of brain plasticity is an emerging area in understanding how the brain functions, and it now includes the concept of neurogenesis. Neurogenesis in the adult brain offers potential hope for future therapies to manage psychiatric disorders and the diseases of human aging such as Alzheimer’s.
3. Viral brain infection
Ependymal cells are the brain’s primary barrier to viral infection. Ependymal cells are susceptible to infection by a wide range of common viruses. They act as a first line of viral defense for the brain. In long-lived organisms such as humans who are exposed repeatedly to viral infections, these cells fight off multiple attacks. It is unknown how repeat viral infection influences the life span of ependymal cells.
Persistent viral infections may be one reason that studies with autopsy material from human brains have failed to discover large populations of ependymal cells lining the lateral ventricles. Similarly, using membrane marker methods, only a small number of mitotically active neuroblasts (developing adult neural stem cells) have been found in the sub-ependymal layer of men and women. However, the neuroblasts present possessed a typical migratory morphology and elongated shape with a small body, which matched that observed in non-human species.
In summary, brain ependymal cells are responsible for cerebrospinal fluid production, protecting the brain against viral infection. and they may or may not protect a reservoir of neural stem cells for the birth of new neurons in adults.
Do you have questions?
Please put your questions in the comment box or send them to me by email at DrReece@MedicalScienceNavigator.com. I read and reply to all comments and email.
If you find this article helpful share it with your fellow students or send it to your favorite social media site by clicking on one of the buttons below.
Further reading
Neurons and Brain’s Other 90% of Cells
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.