Lung gas exchange
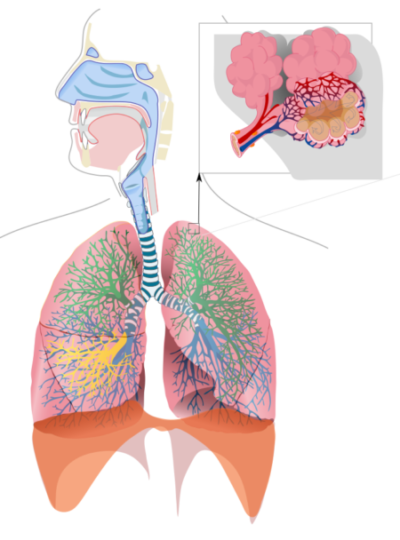
Respiratory system is an important regulator of blood pH, Released to Public Domain by LadyofHats/Wikimedia Commons
There is much recent discussion about the amount of carbon dioxide [CO2] gas that is present in the air we breathe. To understand what this means for lung gas exchange, we need to review the chemistry of the gas laws. Do not panic. It is easy to understand the gas laws if you take it a step at a time. Here are 5 tips for understanding human lung gas exchange.
Tip #1 Atmospheric gases behave as ideal gases
Mathematical models of the dispersion of gas molecules were formulated to define behavior of ideal gases. The atmospheric gasses, oxygen [O2], nitrogen [N2], CO2 and water vapor [H2O] at normal air and body temperature generally follow the rules that explain distribution of ideal gases. So, what is an ideal gas?
All mathematical models are based upon a limited set of assumptions. For the mathematical descriptions of ideal gas behavior, the list of assumptions includes the following.
- Molecules are very far apart relative to their size
- The number of molecules even in small volumes is so large that statistical treatments are valid
- Molecules collide frequently with each other and with the wall of any container enclosing them
- Collisions between molecules are elastic – they bounce off of each other
- Total energy, kinetic plus potential, contained by the gas remains constant
- Molecules are spherical in shape and all contain the same mass, or very near the same
- There are no forces of attraction or repulsion between the gas molecules
- Average kinetic energy of molecules depends on temperature – warmer equals faster moving, higher energy
Tip #2 A simple formula for pulmonary ventilation
The amount of pressure produced by a gas mixture such as Earth’s atmosphere is determined by the number of gas molecules in given volume and the kinetic energy (movement) of those molecules. There are three laws describing the interdependence of gas pressure, gas volume and temperature for ideal gases. They are named Boyle’s Law, Charles’s Law and Gay-Lussac’s Law. Those three laws can be mathematically combined into a simple formula applicable to pulmonary ventilation written as:
P1V1/T1 = P2V2/T2
The important thing to notice about these equations when they are applied to lung physiology is that all three quantities, P = pressure of the gas, V = volume of the container, and T = temperature of the gases, vary as rib cage muscles work to expand and compress lung alveoli.
Alveolar air sacs enlarge increasing the volume available for movement of gas molecules when chest muscles contract lifting the rib cage outward. This causes a drop in gas pressure within the lung to below the pressure of ambient air. Gases are also usually warmed above ambient air temperature in the body and this produces an effect on pressure that counters to a degree the drop due to increased volume alone.
Air flows into and out of the lung as gas pressure in the alveolar sacs fluctuates, because the high kinetic energy of gas molecules causes them to expand into any available container that has a lower gas pressure. As lung volume expands, pressure in the air sacs drops below atmospheric pressure and air molecules move into the lung. As lung volume lessens with relaxation of the respiratory muscles, pressure in the air sacs rises above atmospheric pressure and gas molecules leave the lung through the respiratory passages.
Tip #3 Dalton’s Law of Partial Pressures: O2 in and CO2 out of lung
Pressure differences created by lung expansion and contraction move volumes of air into and out of lungs, but why does that bulk movement of air result in the flow of CO2 and O2 in opposite directions? To explain this another gas law must be introduced, Dalton’s Law of Partial Pressures. This gas law explains best why an increase in carbon dioxide in air may sabotage lung function.
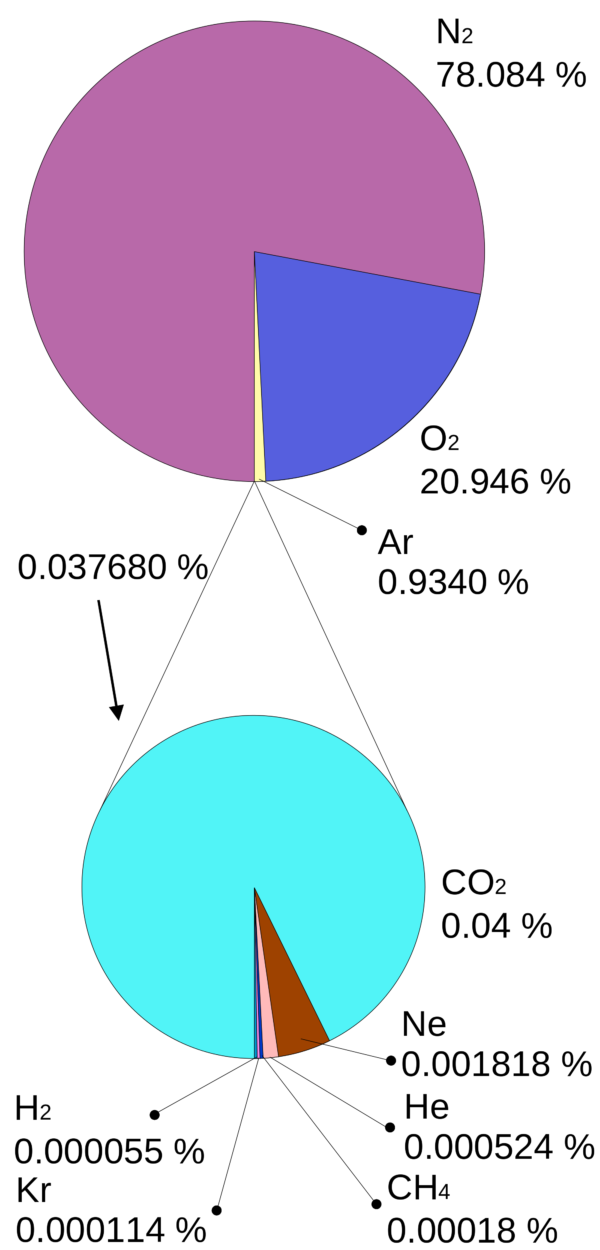
Dalton’s Law of Partial Pressures claims first that when there is a mixture of gases, as in the atmosphere, each gas contributes to the total air pressure in proportion to its percentage in the blend. N2 is responsible for most of the pressure exerted by the atmosphere. As a result, most tissues in the body including blood are saturated with nitrogen. CO2 contributes a very small amount to total air pressure. This is good because CO2 is a waste product of body metabolism and we want it to flow when passages are open toward the atmosphere and out of the body.
Second, Dalton’s Law of Partial Pressures comes to the less obvious conclusion that each gas in a mixture can be treated as if the other gasses were not present for purposes of predicting flow of its molecules from an area of high concentration to an area of low concentration. Pressure brought by each gas in a mixture is not influenced by the other gases in the mixture, because all the gas molecules are too far apart to interact with each other.
This means the pressure difference between O2 in the air and O2 in the alveoli will determine the direction of flow of O2 during pulmonary ventilation. Likewise, the difference between pressure of CO2 in the air and the pressure exerted by CO2 in the alveoli will determine the direction of its flow. Each of these gases inevitably expands away from the area where its own pressure is higher.
Because the body produces CO2 as a waste product that is delivered to lung alveoli by the blood, it is important that it leave the alveoli quickly and easily. The low pressure of CO2 in inhaled air facilitates its movement from alveoli into the volume of air expelled when chest muscles relax.
The same principle applies for O2, but the required path for movement of O2 molecules is the reverse. The body uses O2 very rapidly for extracting chemical energy from food. O2 in air entering alveoli is quickly captured by blood cells moving through the lung. To maintain an adequate supply of O2 for the entire body the O2 pressure of inhaled air must be and is relatively high.
Tip #4 Air pressure quantified
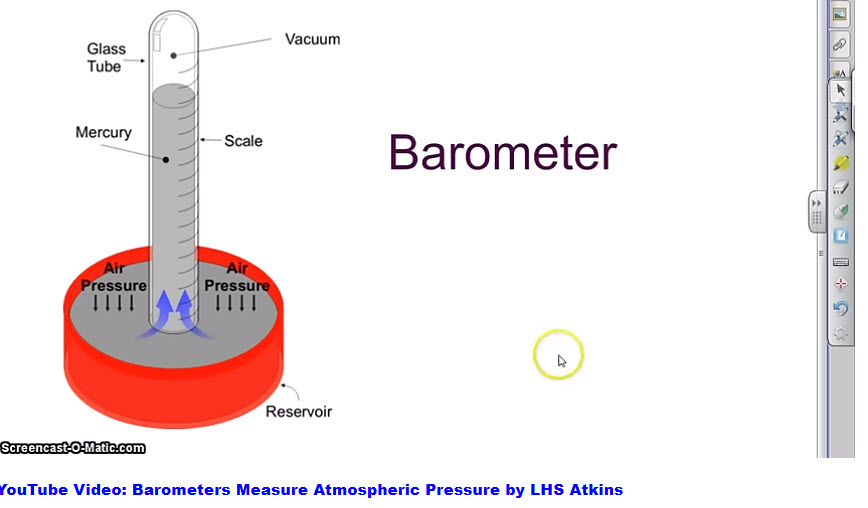
For a mathematical formula to work there must be a numerical value for each of the parameters. Temperature and volume are easy to quantify, but how to quantify atmospheric gas pressure? Learn how by clicking on the image below to start a short video.
By actual measurement at sea level when air temperature is 15°C (59°F) atmospheric pressure is equal to the force needed to drive a column of mercury [Hg] to a height of 760 mm. However, air pressure varies with altitude and can drop at 15,000 feet on a mountain top to about 440 mm Hg. The number of gas molecules is less per unit volume at high altitude, but the percentage distribution of component gases does not change.
Air is composed of the gases shown in the image above plus small quantities of water vapor. Remember Dalton’s Law of Partial Pressures states that when there is a mixture of gases, as in the atmosphere, each gas contributes to the total gas pressure in proportion to its percentage in the blend. If we assume oxygen is 20% of air, its pressure at sea level calculates to be 152 mm Hg (20% of 760 mm Hg) and at 15,000 feet to be about 88 mm Hg (20% of 760 mm Hg). Given that lungs have a limited capacity for volume expansion, the smaller pressure of O2 in mountain air results in a lower pressure of O2 in alveoli and slower delivery of O2 to blood.
Tip # 5 Oxygen solubility in water
The air sacs of the lung, the alveoli, are lined with a watery fluid through which lung gasses must pass when entering and exiting the red cells of the blood. This means solubility of O2 and CO2 in water is an important issue.
O2 and CO2 are nonpolar molecules and therefore do not actually ‘dissolve’ in water like polar molecules. In fact the presence of CO2 in water at its normal partial pressure in air is extremely low.
Moving CO2 through the watery environment of the body requires a complex arrangement of enzyme reactions that involves the bicarbonate buffering system. The nonpolar O2 molecule takes a different path through water. It is able to slip into pockets in the loose lattice of liquid water molecules. The overall size of the pockets between H2O molecules suits the size and shape of O2 molecules.
O2 molecules become trapped in place until the dynamic interaction between H2O molecules causes the pockets to disappear. Once free, O2 pressure pushes the molecules into another pocket always moving them away from where O2 pressure is high to where it is lower. Once O2 reaches an alveolar cell, it dissolves rapidly through the cell’s nonpolar membrane and on into adjacent red blood cells where it binds to hemoglobin.
Now you know one more reason that it is important to keep atmospheric CO2 low, for efficiency of lung gas exchange during pulmonary ventilation.
Do you have questions?
If you want to know more about how the respiratory system works, please put your questions in the comment box or send me an email at DrReece@MedicalScienceNavigator.com. I read and reply to all comments and email.
If you think this description of gas movement to and from the body is helpful, share it with your fellow students or send it to your favorite social media by clicking one of the buttons shown.
Is the chemistry of physiology difficult for you to understand? Check out my book “Physiology: Custom-Designed Chemistry.” It presents a simplified version of all the chemistry you will need to make learning physiology easy. For a look inside click here.
Further reading:
Water’s Chemistry Governs Physiology
Buffering Body Alkalinity and Acidity
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.