Exercise, muscle fatigue, and glycogen
Muscle fatigue, ATP, and glycogen
Originally muscle fatigue was thought to occur because of a shortage of ATP. Surprisingly however, metabolic restoration of ATP to normal level does not immediately ease muscle fatigue.
Muscles remain fatigued and their contractility remains diminished until glycogen stores are also replenished. Glycogen is the storage form of glucose in skeletal muscle.
Mitochondria and sarcoplasmic reticulum
Greater understanding of the mechanism of muscle fatigue came from the discovery of SR-tethered mitochondria. SR-tethered mitochondria are mitochondria tightly bound to sarcoplasmic reticulum. The presence in striated muscle cells of SR-tethered mitochondria is one of many outstanding discoveries in physiology due to unexpected lab assay problems.
Some of the best discoveries in the research laboratory come from analyzing unexpected assay problems. Assay difficulties are common and frustrating in research. But when research protocols persistently produce the same problem, it is time to ask why. What is the system trying to communicate?
Early research efforts to extract mitochondria from skeletal cells failed to produce a clean population of this organelle. The mitochondrial prep was persistently contaminated with sarcoplasmic reticulum.
The presence of sarcoplasmic reticulum contamination in the experimental preparation turned out to be important. It altered the response of the mitochondria to added substrate.
The accepted theory was that mitochondrial enzymes, the energy powerhouse of cells, were regulated simply by substate availability. The altered response to substrate in vitro produced a dilemma. It suggested that mitochondria did not operate independently.
More recent data obtained with intact cells and fluorescent probe molecules discovered mitochondria do not act independently as formerly thought. Local cellular signals allow mitochondria to strategically regulate their rate of ATP production.
SR-tethered mitochondria
The mitochondrial assay contaminant turned out to be an important part of skeletal muscle cells. The ‘contaminating’ membranes in the experimental preparation were tethers tying the sarcoplasmic reticulum (SR) to the mitochondria.
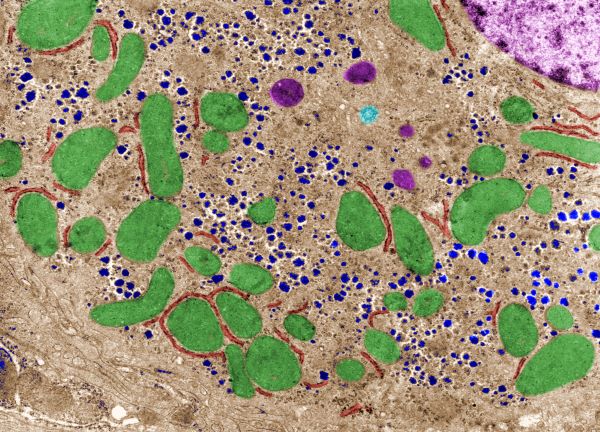
False colour transmission electron microscope (TEM) micrograph showing mitochondria (green), lysosomes (dark pink), glycogen (blue), rough endoplasmic reticulum (red) and a centriole (light blue)., Jose Luis Calvo/Shutterstock.com
Theories of dynamic interactions between mitochondria and cell regulatory mechanisms flourished with the discovery of the tethering structures. Similar tethers are now known to be present in an array of mammalian species.
Only limited details are known about the mechanics of the tethering process. It seems, however, that the Ca++ channels of both organelles are necessary for a stable attachment.
The tethers of mitochondria in cardiac and skeletal muscle include proteins that are critical for the strength of the attachment. The tethers are easily disrupted by controlled protein lysis for in vitro studies.
Mitochondria and muscle contraction
Striated muscle contains a large population of mitochondria. Mitochondria occupy about 40% of cardiac and 20% of skeletal muscle cell volume.
In both types of striated muscle, mitochondria are located near to the Ca++ release units of the sarcoplasmic reticulum, which are activated during muscle contraction.
Some of the Ca++ released during muscle contraction enters the mitochondria. Ca++ entering the mitochondria signals the need for an increased rate of ATP production.
The close arrangement of the mitochondria and the sarcoplasmic reticulum Ca++ channels also benefit sarcoplasmic Ca++ re-uptake. Re-uptake pumps, like those of the sarcoplasmic reticulum, which move Ca++ against its concentration gradient, require a large amount of local ATP.
Molecular mechanisms of muscle fatigue
With discovery of the close structural contact between mitochondria and the sarcoplasmic reticulum, additional theories emerged to explain the molecular mechanism of muscle fatigue.
One theory is that Ca++ channels of the SR-tethered mitochondria play a role in regulation of the enzymes of the nearby glycogen granule complexes. The glycogen/enzyme complexes are shown as blue granules in the electron microscope picture above.
Glycogen granule, released to public domain by owner Häggström, Mikael (2014). “Medical gallery of Mikael Häggström 2014”/Wikimedia Commons
Each glycogen granule in muscle exists in a complex with the set of enzymes needed to alternately synthesize it and break it down into glucose molecules. In muscle, it is likely that glucose is metabolized near the glycogen granule that released it. Muscle fibers, because of their highly ordered structure, do not depend upon diffusion of glucose within the cell.
Glycogen is allocated to three physically distant pools within muscle fibers. One pool, containing about 12% to 15% of the granules, is located directly under the sarcolemma, the cell membrane.
The largest pool of glycogen, about 75% of the cell’s total glycogen resides between myofibrils near SR-tethered mitochondria. The fiber’s remaining glycogen is found between myofibrils near the Z-lines.
After exercise that produces fatigue, each of the glycogen pools is about 70% to 90% depleted. But quantitatively the largest amount of remaining glycogen is found near the SR-tethered mitochondria.
Possibly it is this pool of glycogen that is used for the energy required to restore normal function of the nearby sarcoplasmic Ca++ channels. Testing such theories may require more detailed study of SR-tethered mitochondria in assays containing ‘contaminant’ membranes.
Contractile proteins of muscle
If you are struggling with the mechanics of striated muscle cell contraction, here is a helpful video from Interactive Biology at YouTube by Leslie Samuel that explains the contractile proteins of muscle and how they work together.
Further reading:
Muscle Origins, Insertions and Levers
Do you have questions?
Send me an email with your questions at DrReece@MedicalScienceNavigator.com or put them in the comment box. I always read my mail and answer it. Please share this article with your fellow students taking anatomy and physiology by clicking your favorite social media button.
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.


