Chemical properties of water
Water is physiology’s most important molecule. The chemical properties of water in living systems continue to be an active area of scientific research. Scientists are still discovering unique aspects of water’s molecular nature when it is confined to biologic spaces.
What is a chemical?
To answer what is a chemical, it may be best to answer first what is NOT a chemical? How did the word chemical get such a bad reputation? Plain old, one hundred percent pure, ordinary water is a strong chemical. There is a lot of confusion in the popular press about what constitutes a chemical. Even chemistry textbooks have been known to define chemical substances as “any material with a definite chemical composition.”
Much of what we read in product ads misleads us about the definition of a chemical. “Chemical-free” is used in advertising to imply the safety of a product. It leaves us with the impression that there is something not natural and bad about chemicals. Yet the word chemical is a synonym for matter. Matter makes up the universe. Humans, being part of the universe, are made up of chemicals. And, the primary chemical that controls the dynamic we call physiology (or life) is water. So, let’s talk about the chemical properties of water.
Structure of atoms
To understand water’s chemistry, we first must learn a bit about atoms. This is because water is composed of two types of atoms – hydrogen and oxygen.
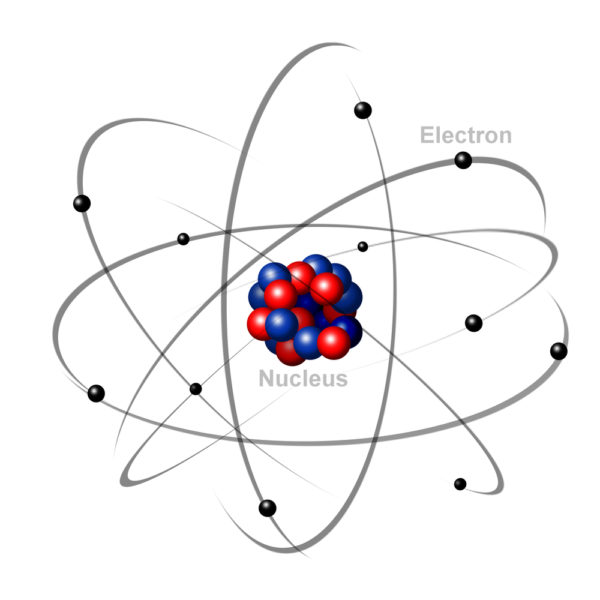
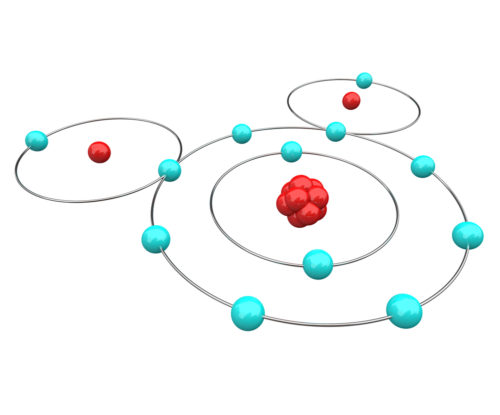
For our purposes we can think of atoms as being made up of three components, protons, electrons, and neutrons. The number of protons that an atom has determines the unique nature of the matter formed by that atom. Hydrogen matter has one proton. Oxygen matter has eight protons. The number of electrons in each atom always equals the number of protons.
The electrical charge of each atom’s protons (positive) is strong enough to hold its own electrons (negative charges) in circling orbitals close by. But electrons move around the nucleus so rapidly that it is not physically possible to tell where any one electron is at any given point in time.
To get around the issue of electron location, scientists have agreed to predict the movement of electrons based upon mathematical probability equations. Probability equations calculate that an atom’s electrons form layers – each layer containing an optimal number of electrons. The result of assigning an optimal number of electrons to each layer is that most atoms end up with an outermost orbital layer that has either more or less electrons than is optimal.
Structure of water
The structure of water provides it some unique chemical properties for a small molecule. Sharing of electrons by two or more atoms is a common method for satisfying the need for an optimal number of electrons in the outermost orbital layers of all involved. Links called covalent molecular bonds occur between atoms when the outermost electron orbital of all participants becomes complete by the formation of a hybrid orbital.
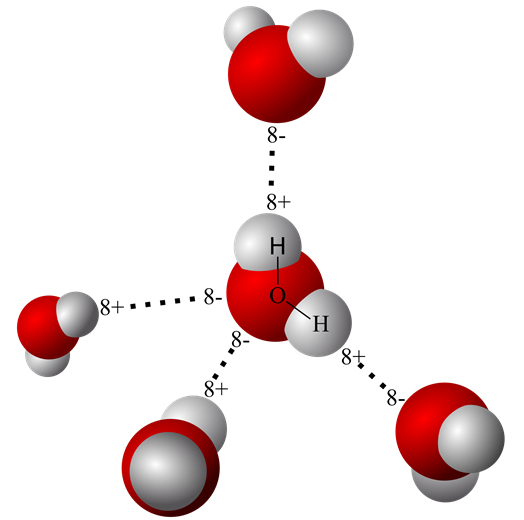
In the case of the water molecule, oxygen shares its outer orbital layer with two electrons, one each from two hydrogen atoms. A simplified drawing of the covalent molecular bonds of water is pictured below.
The sharing of electrons in the water molecule, however, is not equal between oxygen and hydrogen. Oxygen’s eight protons exert a much greater pulling force on electrons in the outer orbital than do hydrogen’s protons. Unequal sharing of electrons creates a polar water molecule with a partial negative charge around the oxygen atom – where the electrons are spending most of their time. Hydrogen’s electrons spend much less time near the hydrogen proton. This creates a partial positive charge at the hydrogen end of the molecule where the proton’s charge dominates.
The partial charges on opposite ends of the water molecules allow them to enter short-lived, weak electrostatic bonds called hydrogen bonds. Hydrogen bonds form between liquid water molecules and between liquid water molecules and other positively or negatively charged molecules.
Hydrophilic vs. hydrophobic molecules
The terms hydrophilic and hydrophobic derive from Latin terms. Hydro- means water, and -philic refers to loving. Some molecules love their interaction with water and are called hydrophilic. Phobic refers to aversion or disliking. Hydrophobic molecules avoid mixing with water.
The ability of polar water molecules to form hydrogen bonds is used to explain water’s ability to take charged molecules into solution. If water molecules encounter other molecules with a full or partial charge, they will be electrically attracted to them and surround them. Hydrophilic molecules occupy the fluid compartments of the body.
Other molecules which have an aversion to water – the hydrophobic molecules – form biologic membranes. Physiology can be thought of as an organized crosstalk between watery compartments of hydrophilic molecules separated by biologic membranes composed of hydrophobic molecules.
For a complete beginner’s guide to water’s chemistry and its effect on human physiology, check out my easy read book “Physiology: Custom-Designed Chemistry.” It is available both as an e-book and as a paperback book. Click here for a look inside.
Further reading
Do you have questions?
Please put your questions in the comment box or send them to me by email at DrReece@MedicalScienceNavigator.com. I read and reply to all comments and email.
If you find this article helpful share it with your fellow students or send it to your favorite social media site by clicking on one of the buttons below.
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.